Japanese rapid/living recommendations on drug management for COVID-19
- PMID: 34178358
- PMCID: PMC8209876
- DOI: 10.1002/ams2.664
Japanese rapid/living recommendations on drug management for COVID-19
Abstract
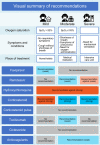
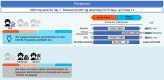
The coronavirus disease (COVID-19) has spread worldwide since early 2020, and there are still no signs of resolution. The Japanese Clinical Practice Guidelines for the Management of Sepsis and Septic Shock (J-SSCG) 2020 Special Committee created the Japanese rapid/living recommendations on drug management for COVID-19 using the experience of creating the J-SSCGs. The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach was used to determine the certainty of the evidence and strength of the recommendations. The first edition of this guideline was released on 9 September, 2020, and this document is the revised edition (version 3.1) (released 30 March, 2021). Clinical questions (CQs) were set for the following seven drugs: favipiravir (CQ1), remdesivir (CQ2), hydroxychloroquine (CQ3), corticosteroids (CQ4), tocilizumab (CQ5), ciclesonide (CQ6), and anticoagulants (CQ7). Favipiravir is recommended for patients with mild COVID-19 not requiring supplemental oxygen (GRADE 2C); remdesivir for moderate COVID-19 patients requiring supplemental oxygen/hospitalization (GRADE 2B). Hydroxychloroquine is not recommended for all COVID-19 patients (GRADE 1B). Corticosteroids are recommended for moderate COVID-19 patients requiring supplemental oxygen/hospitalization (GRADE 1B) and severe COVID-19 patients requiring ventilator management/intensive care (GRADE 1A); however, their use is not recommended for mild COVID-19 patients not requiring supplemental oxygen (GRADE 1B). Tocilizumab is recommended for moderate COVID-19 patients requiring supplemental oxygen/hospitalization (GRADE 2B). Anticoagulant therapy is recommended for moderate COVID-19 patients requiring supplemental oxygen/hospitalization and severe COVID-19 patients requiring ventilator management/intensive care (GRADE 2C). We hope that these clinical practice guidelines will aid medical professionals involved in the care of COVID-19 patients.
Keywords: Coronavirus; GRADE approach; SIRS‐CoV‐2; evidence‐based medicine; practice guideline.
© 2021 The Authors. Acute Medicine & Surgery published by John Wiley & Sons Australia, Ltd on behalf of Japanese Association for Acute Medicine.
Conflict of interest statement
Approval of the research protocol: N/A. Informed consent: N/A. Registry and the registration no. of the study/trial: N/A. Animal studies: N/A. Conflict of interest: The Japanese Society of Intensive Care Medicine and the Japanese Association for Acute Medicine submitted this conflict of interest (COI) disclosure jointly, based on the same policy issued by the Japanese Association of Medical Sciences. In accordance with these guidelines, organizations are only required to disclose COI that relate to associated companies or for‐profit organizations as financial COI. We asked all members to submit their financial and academic COI for the past three years (2017–2019), in accordance with the current policy, shown in Document S1.
Figures





References
-
- Fitch KBS, Aguilar MD, Burnand B, et al. The Rand/UCLA Appropriateness Method User's Manual. Santa Monica, CA: RAND Corporation, 2001.
-
- Clinical Management of Patients with COVID‐19: A guide for front‐line healthcare workers.
-
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2019; 366: l4898. - PubMed

