Proteomic Analysis of Rhesus Macaque Brain Explants Treated With Borrelia burgdorferi Identifies Host GAP-43 as a Potential Factor Associated With Lyme Neuroborreliosis
- PMID: 34178719
- PMCID: PMC8224226
- DOI: 10.3389/fcimb.2021.647662
Proteomic Analysis of Rhesus Macaque Brain Explants Treated With Borrelia burgdorferi Identifies Host GAP-43 as a Potential Factor Associated With Lyme Neuroborreliosis
Abstract
Background: Lyme neuroborreliosis (LNB) is one of the most dangerous manifestations of Lyme disease, but the pathogenesis and inflammatory mechanisms are not fully understood.
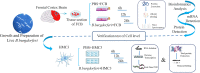
Methods: Cultured explants from the frontal cortex of rhesus monkey brain (n=3) were treated with live Borrelia burgdorferi (Bb) or phosphate-buffered saline (PBS) for 6, 12, and 24 h. Total protein was collected for sequencing and bioinformatics analysis. In addition, changes in protein expression in the explants over time following Bb treatment were screened.
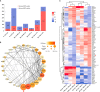
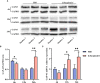
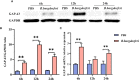
Results: We identified 1237 differentially expressed proteins (DEPs; fold change ≥1.5 or ≤0.67, P-value ≤0.05). One of these, growth-associated protein 43 (GAP-43), was highly expressed at all time points in the explants. The results of the protein-protein interaction network analysis of DEPs suggested that GAP-43 plays a role in the neuroinflammation associated with LNB. In HMC3 cells incubated with live Bb or PBS for 6, 12, and 24 h, real-time PCR and western blot analyses confirmed the increase of GAP-43 mRNA and protein, respectively.
Conclusions: Elevated GAP-43 expression is a potential marker for LNB that may be useful for diagnosis or treatment.
Keywords: Borrelia burgdorferi; GAP-43; HMC3; lyme neuroborreliosis; neuroinflammation; proteomic analysis.
Copyright © 2021 Li, Luo, Chen, Cao, Xu, Zhang, Yue, Fan, Chen, Liu, Ma, Tao, Peng, Dong, Li, Luo, Kong, Zhou, Wen, Liu and Bao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Comprehensive analyses of transcriptomes induced by Lyme spirochete infection to CNS model system.Infect Genet Evol. 2022 Sep;103:105349. doi: 10.1016/j.meegid.2022.105349. Epub 2022 Aug 12. Infect Genet Evol. 2022. PMID: 35964914
-
Comparative proteomics profiling revealed the involvement of GRB2-ROCK2 axis in Lyme neuroborreliosis caused by Borrelia Burgdorferi.J Cell Mol Med. 2022 Apr;26(8):2312-2321. doi: 10.1111/jcmm.17253. Epub 2022 Feb 25. J Cell Mol Med. 2022. PMID: 35212166 Free PMC article.
-
Rhesus Brain Transcriptomic Landscape in an ex vivo Model of the Interaction of Live Borrelia Burgdorferi With Frontal Cortex Tissue Explants.Front Neurosci. 2019 Jun 28;13:651. doi: 10.3389/fnins.2019.00651. eCollection 2019. Front Neurosci. 2019. PMID: 31316336 Free PMC article.
-
The rhesus model of Lyme neuroborreliosis.Immunol Rev. 2001 Oct;183:186-204. doi: 10.1034/j.1600-065x.2001.1830115.x. Immunol Rev. 2001. PMID: 11782257 Review.
-
Pathogenesis of neuroborreliosis--lessons from a monkey model.Wien Klin Wochenschr. 1998 Dec 23;110(24):870-3. Wien Klin Wochenschr. 1998. PMID: 10048168 Review.
Cited by
-
A key protein from Borrelia burgdorferi could stimulate cytokines in human microglial cells and inhibitory effects of Cucurbitacin IIa.IBRO Neurosci Rep. 2023 Nov 13;15:376-385. doi: 10.1016/j.ibneur.2023.11.004. eCollection 2023 Dec. IBRO Neurosci Rep. 2023. PMID: 38046885 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

