miR-548d-3p Alters Parasite Growth and Inflammation in Leishmania (Viannia) braziliensis Infection
- PMID: 34178725
- PMCID: PMC8224172
- DOI: 10.3389/fcimb.2021.687647
miR-548d-3p Alters Parasite Growth and Inflammation in Leishmania (Viannia) braziliensis Infection
Abstract
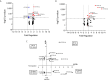
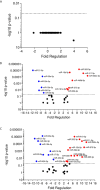
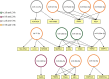
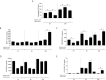
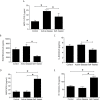
American Tegumentary Leishmaniasis (ATL) is an endemic disease in Latin America, mainly caused in Brazil by Leishmania (Viannia) braziliensis. Clinical manifestations vary from mild, localized cutaneous leishmaniasis (CL) to aggressive mucosal disease. The host immune response strongly determines the outcome of infection and pattern of disease. However, the pathogenesis of ATL is not well understood, and host microRNAs (miRNAs) may have a role in this context. In the present study, miRNAs were quantified using qPCR arrays in human monocytic THP-1 cells infected in vitro with L. (V.) braziliensis promastigotes and in plasma from patients with ATL, focusing on inflammatory response-specific miRNAs. Patients with active or self-healed cutaneous leishmaniasis patients, with confirmed parasitological or immunological diagnosis, were compared with healthy controls. Computational target prediction of significantly-altered miRNAs from in vitro L. (V.) braziliensis-infected THP-1 cells revealed predicted targets involved in diverse pathways, including chemokine signaling, inflammatory, cellular proliferation, and tissue repair processes. In plasma, we observed distinct miRNA expression in patients with self-healed and active lesions compared with healthy controls. Some miRNAs dysregulated during THP-1 in vitro infection were also found in plasma from self-healed patients, including miR-548d-3p, which was upregulated in infected THP-1 cells and in plasma from self-healed patients. As miR-548d-3p was predicted to target the chemokine pathway and inflammation is a central to the pathogenesis of ATL, we evaluated the effect of transient transfection of a miR-548d-3p inhibitor on L. (V.) braziliensis infected-THP-1 cells. Inhibition of miR-548d-3p reduced parasite growth early after infection and increased production of MCP1/CCL2, RANTES/CCL5, and IP10/CXCL10. In plasma of self-healed patients, MCP1/CCL2, RANTES/CCL5, and IL-8/CXCL8 concentrations were significantly decreased and MIG/CXCL9 and IP-10/CXCL10 increased compared to patients with active disease. These data suggest that by modulating miRNAs, L. (V.) braziliensis may interfere with chemokine production and hence the inflammatory processes underpinning lesion resolution. Our data suggest miR-548d-3p could be further evaluated as a prognostic marker for ATL and/or as a host-directed therapeutic target.
Keywords: Leishmania braziliensis; THP-1 cells; active cutaneous leishmaniasis; microRNA; pathogenesis; self-healed cutaneous leishmaniasis.
Copyright © 2021 Souza, Ramos-Sanchez, Muxel, Lagos, Reis, Pereira, Brito, Zampieri, Kaye, Floeter-Winter and Goto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
miR-548d-3p Is Up-Regulated in Human Visceral Leishmaniasis and Suppresses Parasite Growth in Macrophages.Front Cell Infect Microbiol. 2022 Feb 10;12:826039. doi: 10.3389/fcimb.2022.826039. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35265535 Free PMC article.
-
The miRNA 361-3p, a Regulator of GZMB and TNF Is Associated With Therapeutic Failure and Longer Time Healing of Cutaneous Leishmaniasis Caused by L. (viannia) braziliensis.Front Immunol. 2018 Nov 14;9:2621. doi: 10.3389/fimmu.2018.02621. eCollection 2018. Front Immunol. 2018. PMID: 30487794 Free PMC article.
-
MicroRNAs regulating macrophages infected with Leishmania L. (V.) Braziliensis isolated from different clinical forms of American tegumentary leishmaniasis.Front Immunol. 2023 Dec 7;14:1280949. doi: 10.3389/fimmu.2023.1280949. eCollection 2023. Front Immunol. 2023. PMID: 38143766 Free PMC article.
-
Development of real-time PCR assays for evaluation of immune response and parasite load in golden hamster (Mesocricetus auratus) infected by Leishmania (Viannia) braziliensis.Parasit Vectors. 2016 Jun 27;9(1):361. doi: 10.1186/s13071-016-1647-6. Parasit Vectors. 2016. PMID: 27350537 Free PMC article.
-
Protection and Pathology in Leishmania braziliensis Infection.Pathogens. 2022 Apr 14;11(4):466. doi: 10.3390/pathogens11040466. Pathogens. 2022. PMID: 35456141 Free PMC article. Review.
Cited by
-
Recent advances in the development and clinical application of miRNAs in infectious diseases.Noncoding RNA Res. 2024 Sep 2;10:41-54. doi: 10.1016/j.ncrna.2024.09.005. eCollection 2025 Feb. Noncoding RNA Res. 2024. PMID: 39296638 Free PMC article. Review.
-
miR-548d-3p Is Up-Regulated in Human Visceral Leishmaniasis and Suppresses Parasite Growth in Macrophages.Front Cell Infect Microbiol. 2022 Feb 10;12:826039. doi: 10.3389/fcimb.2022.826039. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35265535 Free PMC article.
-
The pathogenicity and virulence of Leishmania - interplay of virulence factors with host defenses.Virulence. 2022 Dec;13(1):903-935. doi: 10.1080/21505594.2022.2074130. Virulence. 2022. PMID: 35531875 Free PMC article. Review.
-
Early Leishmania infectivity depends on miR-372/373/520d family-mediated reprogramming of polyamines metabolism in THP-1-derived macrophages.Sci Rep. 2024 Jan 10;14(1):996. doi: 10.1038/s41598-024-51511-y. Sci Rep. 2024. PMID: 38200138 Free PMC article.
-
Editorial: Immunology and immunopathogenesis of human leishmaniasis.Front Cell Infect Microbiol. 2022 Oct 14;12:1055221. doi: 10.3389/fcimb.2022.1055221. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36310861 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

