Sirtuin Control of Mitochondrial Dysfunction, Oxidative Stress, and Inflammation in Chagas Disease Models
- PMID: 34178728
- PMCID: PMC8221535
- DOI: 10.3389/fcimb.2021.693051
Sirtuin Control of Mitochondrial Dysfunction, Oxidative Stress, and Inflammation in Chagas Disease Models
Abstract
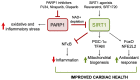
Trypanosoma cruzi is a digenetic parasite that requires triatomines and mammalian host to complete its life cycle. T. cruzi replication in mammalian host induces immune-mediated cytotoxic proinflammatory reactions and cellular injuries, which are the common source of reactive oxygen species (ROS) and reactive nitrogen species (RNS) during the acute parasitemic phase. Mitochondrial dysfunction of electron transport chain has been proposed as a major source of superoxide release in the chronic phase of infection, which renders myocardium exposed to sustained oxidative stress and contributes to Chagas disease pathology. Sirtuin 1 (SIRT1) is a class III histone deacetylase that acts as a sensor of redox changes and shapes the mitochondrial metabolism and inflammatory response in the host. In this review, we discuss the molecular mechanisms by which SIRT1 can potentially improve mitochondrial function and control oxidative and inflammatory stress in Chagas disease.
Keywords: Chagas disease; mitochondrial dysfunction; peroxisome proliferator-activated receptor gamma coactivator 1; reactive oxygen species; sirtuin.
Copyright © 2021 Wan and Garg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
SIRT1-PGC1α-NFκB Pathway of Oxidative and Inflammatory Stress during Trypanosoma cruzi Infection: Benefits of SIRT1-Targeted Therapy in Improving Heart Function in Chagas Disease.PLoS Pathog. 2016 Oct 20;12(10):e1005954. doi: 10.1371/journal.ppat.1005954. eCollection 2016 Oct. PLoS Pathog. 2016. PMID: 27764247 Free PMC article.
-
Dual and Opposite Roles of Reactive Oxygen Species (ROS) in Chagas Disease: Beneficial on the Pathogen and Harmful on the Host.Oxid Med Cell Longev. 2020 Dec 10;2020:8867701. doi: 10.1155/2020/8867701. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33376582 Free PMC article. Review.
-
Origin of Monocytes/Macrophages Contributing to Chronic Inflammation in Chagas Disease: SIRT1 Inhibition of FAK-NFκB-Dependent Proliferation and Proinflammatory Activation of Macrophages.Cells. 2019 Dec 28;9(1):80. doi: 10.3390/cells9010080. Cells. 2019. PMID: 31905606 Free PMC article.
-
The Oxidative Stress and Chronic Inflammatory Process in Chagas Disease: Role of Exosomes and Contributing Genetic Factors.Oxid Med Cell Longev. 2021 Dec 23;2021:4993452. doi: 10.1155/2021/4993452. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34976301 Free PMC article. Review.
-
Manganese superoxide dismutase deficiency exacerbates the mitochondrial ROS production and oxidative damage in Chagas disease.PLoS Negl Trop Dis. 2018 Jul 25;12(7):e0006687. doi: 10.1371/journal.pntd.0006687. eCollection 2018 Jul. PLoS Negl Trop Dis. 2018. PMID: 30044789 Free PMC article.
Cited by
-
Nicotinamide Adenine Dinucleotide (NAD)-Dependent Protein Deacetylase, Sirtuin, as a Biomarker of Healthy Life Expectancy: A Mini- Review.Curr Aging Sci. 2025;18(2):95-101. doi: 10.2174/0118746098319674240827104612. Curr Aging Sci. 2025. PMID: 39377386 Free PMC article. Review.
-
Exploring Synergistic Inhibition of Inflammatory and Antioxidant Potential: Integrated In Silico and In Vitro Analyses of Garcinia mangostana, Curcuma comosa, and Acanthus ebracteatus.Adv Pharmacol Pharm Sci. 2024 Sep 18;2024:8584015. doi: 10.1155/2024/8584015. eCollection 2024. Adv Pharmacol Pharm Sci. 2024. PMID: 39328582 Free PMC article.
-
Neospora caninum infection induced mitochondrial dysfunction in caprine endometrial epithelial cells via downregulating SIRT1.Parasit Vectors. 2022 Aug 1;15(1):274. doi: 10.1186/s13071-022-05406-4. Parasit Vectors. 2022. PMID: 35915458 Free PMC article.
-
Exploring the beneficial effects of GHK-Cu on an experimental model of colitis and the underlying mechanisms.Front Pharmacol. 2025 Jul 2;16:1551843. doi: 10.3389/fphar.2025.1551843. eCollection 2025. Front Pharmacol. 2025. PMID: 40672369 Free PMC article.
-
Mitochondrial Metabolism in Major Depressive Disorder: From Early Diagnosis to Emerging Treatment Options.J Clin Med. 2024 Mar 17;13(6):1727. doi: 10.3390/jcm13061727. J Clin Med. 2024. PMID: 38541952 Free PMC article. Review.
References
-
- Alvarez M. N., Peluffo G., Piacenza L., Radi R. (2011). Intraphagosomal Peroxynitrite as a Macrophage-Derived Cytotoxin Against Internalized Trypanosoma Cruzi: Consequences for Oxidative Killing and Role of Microbial Peroxiredoxins in Infectivity. J. Biol. Chem. 286, 6627–6640. 10.1074/jbc.M110.167247 - DOI - PMC - PubMed
-
- Amat R., Planavila A., Chen S. L., Iglesias R., Giralt M., Villarroya F. (2009). SIRT1 Controls the Transcription of the Peroxisome Proliferator-Activated Receptor-Gamma Co-activator-1alpha (PGE-1alpha) Gene in Skeletal Muscle Through the PGC-1alpha Autoregulatory Loop and Interaction With Myod. J. Biol. Chem. 284, 21872–21880. 10.1074/jbc.M109.022749 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

