Cell Death and Exosomes Regulation After Myocardial Infarction and Ischemia-Reperfusion
- PMID: 34179002
- PMCID: PMC8220218
- DOI: 10.3389/fcell.2021.673677
Cell Death and Exosomes Regulation After Myocardial Infarction and Ischemia-Reperfusion
Abstract
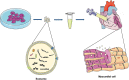
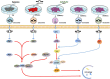
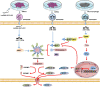
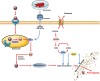
Cardiovascular disease (CVD) is the leading cause of death in the global population, accounting for about one-third of all deaths each year. Notably, with CVDs, myocardial damages result from myocardial infarction (MI) or cardiac arrhythmias caused by interrupted blood flow. Significantly, in the process of MI or myocardial ischemic-reperfusion (I/R) injury, both regulated and non-regulated cell death methods are involved. The critical factor for patients' prognosis is the infarct area's size, which determines the myocardial cells' survival. Cell therapy for MI has been a research hotspot in recent years; however, exosomes secreted by cells have attracted much attention following shortcomings concerning immunogens. Exosomes are extracellular vesicles containing several biologically active substances such as lipids, nucleic acids, and proteins. New evidence suggests that exosomes play a crucial role in regulating cell death after MI as exosomes of various stem cells can participate in the cell damage process after MI. Hence, in the review herein, we focused on introducing various cell-derived exosomes to reduce cell death after MI by regulating the cell death pathway to understand myocardial repair mechanisms better and provide a reference for clinical treatment.
Keywords: apoptosis; autophagy-dependent death; exosomes; ferroptosis; microRNA; myocardial infarction; myocardial protection; pyroptosis.
Copyright © 2021 Wu, Iroegbu, Peng, Guo, Yang and Fan.
Conflict of interest statement
JG was employed by the company Hunan Fangsheng Pharmaceutical Co. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Exosomes as a Cell-free Therapy for Myocardial Injury Following Acute Myocardial Infarction or Ischemic Reperfusion.Aging Dis. 2022 Dec 1;13(6):1770-1786. doi: 10.14336/AD.2022.0416. eCollection 2022 Dec 1. Aging Dis. 2022. PMID: 36465167 Free PMC article. Review.
-
The pivotal roles of exosomes derived from endogenous immune cells and exogenous stem cells in myocardial repair after acute myocardial infarction.Theranostics. 2021 Jan 1;11(3):1046-1058. doi: 10.7150/thno.53326. eCollection 2021. Theranostics. 2021. PMID: 33391520 Free PMC article. Review.
-
Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways.Cell Physiol Biochem. 2017;43(1):52-68. doi: 10.1159/000480317. Epub 2017 Aug 25. Cell Physiol Biochem. 2017. PMID: 28848091
-
MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction.Eur Rev Med Pharmacol Sci. 2020 Oct;24(19):10107-10117. doi: 10.26355/eurrev_202010_23230. Eur Rev Med Pharmacol Sci. 2020. PMID: 33090418
-
Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury.Stem Cell Res. 2013 May;10(3):301-12. doi: 10.1016/j.scr.2013.01.002. Epub 2013 Jan 14. Stem Cell Res. 2013. PMID: 23399448
Cited by
-
Communication Regarding the Myocardial Ischemia/Reperfusion and Cognitive Impairment: A Narrative Literature Review.J Alzheimers Dis. 2024;97(4):1545-1570. doi: 10.3233/JAD-230886. J Alzheimers Dis. 2024. PMID: 38277294 Free PMC article. Review.
-
Inhibition of microRNA-122 alleviates pyroptosis by targeting dual-specificity phosphatase 4 in myocardial ischemia/reperfusion injury.Heliyon. 2023 Jul 13;9(7):e18238. doi: 10.1016/j.heliyon.2023.e18238. eCollection 2023 Jul. Heliyon. 2023. PMID: 37539226 Free PMC article.
-
Therapeutic application of adipose-derived stromal vascular fraction in myocardial infarction.iScience. 2024 Apr 18;27(5):109791. doi: 10.1016/j.isci.2024.109791. eCollection 2024 May 17. iScience. 2024. PMID: 38736548 Free PMC article. Review.
-
The outcomes of three different techniques of coronary artery bypass grafting: On-pump arrested heart, on-pump beating heart, and off-pump.PLoS One. 2023 May 31;18(5):e0286510. doi: 10.1371/journal.pone.0286510. eCollection 2023. PLoS One. 2023. PMID: 37256890 Free PMC article.
-
Ferroptosis and protein translation: emerging perspectives in the research of myocardial infraction.Front Cardiovasc Med. 2025 May 2;12:1592333. doi: 10.3389/fcvm.2025.1592333. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40384965 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

