The Calcium Binding Protein S100A11 and Its Roles in Diseases
- PMID: 34179021
- PMCID: PMC8226020
- DOI: 10.3389/fcell.2021.693262
The Calcium Binding Protein S100A11 and Its Roles in Diseases
Abstract
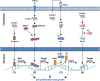
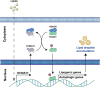
The calcium binding protein S100 family in humans contains 21 known members, with each possessing a molecular weight between 10 and 14 kDa. These proteins are characterized by a unique helix-loop-helix EF hand motif, and often form dimers and multimers. The S100 family mainly exists in vertebrates and exerts its biological functions both inside cells as a calcium sensor/binding protein, as well as outside cells. S100A11, a member of the S100 family, may mediate signal transduction in response to internal or external stimuli and it plays various roles in different diseases such as cancers, metabolic disease, neurological diseases, and vascular calcification. In addition, it can function as chemotactic agent in inflammatory disease. In this review, we first detail the discovery of S100 proteins and their structural features, and then specifically focus on the tissue and organ expression of S100A11. We also summarize its biological activities and roles in different disease and signaling pathways, providing an overview of S100A11 research thus far.
Keywords: S100 proteins; S100A11; diseases; protein interaction; signaling pathways.
Copyright © 2021 Zhang, Zhu, Miao and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
S100A11: diverse function and pathology corresponding to different target proteins.Cell Biochem Biophys. 2009;55(3):117-26. doi: 10.1007/s12013-009-9061-8. Epub 2009 Aug 1. Cell Biochem Biophys. 2009. PMID: 19649745 Review.
-
Identification of a novel interaction between the Ca(2+)-binding protein S100A11 and the Ca(2+)- and phospholipid-binding protein annexin A6.Am J Physiol Cell Physiol. 2007 Apr;292(4):C1417-30. doi: 10.1152/ajpcell.00439.2006. Epub 2006 Dec 27. Am J Physiol Cell Physiol. 2007. PMID: 17192283
-
Insights into S100 target specificity examined by a new interaction between S100A11 and annexin A2.Biochemistry. 2006 Dec 12;45(49):14695-705. doi: 10.1021/bi061754e. Biochemistry. 2006. PMID: 17144662
-
Formation of monomeric S100B and S100A11 proteins at low ionic strength.Biochemistry. 2009 Mar 10;48(9):1954-63. doi: 10.1021/bi802086a. Biochemistry. 2009. PMID: 19216510
-
S100 proteins in the epidermis.J Invest Dermatol. 2004 Jul;123(1):23-33. doi: 10.1111/j.0022-202X.2004.22719.x. J Invest Dermatol. 2004. PMID: 15191538 Review.
Cited by
-
FLIM-FRET-based analysis of S100A11/annexin interactions in living cells.FEBS Open Bio. 2024 Apr;14(4):626-642. doi: 10.1002/2211-5463.13782. Epub 2024 Feb 26. FEBS Open Bio. 2024. PMID: 38408765 Free PMC article.
-
ANXA2 in cancer: aberrant regulation of tumour cell apoptosis and its immune interactions.Cell Death Discov. 2025 Apr 15;11(1):174. doi: 10.1038/s41420-025-02469-x. Cell Death Discov. 2025. PMID: 40234383 Free PMC article. Review.
-
Lactylation-related gene signatures identify glioma molecular subtypes with prognostic, immunological, and therapeutic implications.Front Oncol. 2025 Jul 16;15:1613423. doi: 10.3389/fonc.2025.1613423. eCollection 2025. Front Oncol. 2025. PMID: 40740857 Free PMC article.
-
S100A11 Promotes Metastasis via AKT and ERK Signaling Pathways and Has a Diagnostic Role in Hepatocellular Carcinoma.Int J Med Sci. 2023 Jan 31;20(3):318-328. doi: 10.7150/ijms.80503. eCollection 2023. Int J Med Sci. 2023. PMID: 36860671 Free PMC article.
-
Surfaceome analysis of extracellular vesicles from senescent cells uncovers uptake repressor DPP4.Proc Natl Acad Sci U S A. 2023 Oct 24;120(43):e2219801120. doi: 10.1073/pnas.2219801120. Epub 2023 Oct 20. Proc Natl Acad Sci U S A. 2023. PMID: 37862381 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

