Urinary Extracellular Vesicles for Renal Tubular Transporters Expression in Patients With Gitelman Syndrome
- PMID: 34179047
- PMCID: PMC8219937
- DOI: 10.3389/fmed.2021.679171
Urinary Extracellular Vesicles for Renal Tubular Transporters Expression in Patients With Gitelman Syndrome
Abstract
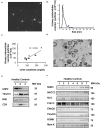
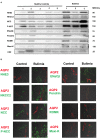
Background: The utility of urinary extracellular vesicles (uEVs) to faithfully represent the changes of renal tubular protein expression remains unclear. We aimed to evaluate renal tubular sodium (Na+) or potassium (K+) associated transporters expression from uEVs and kidney tissues in patients with Gitelman syndrome (GS) caused by inactivating mutations in SLC12A3. Methods: uEVs were isolated by ultracentrifugation from 10 genetically-confirmed GS patients. Membrane transporters including Na+-hydrogen exchanger 3 (NHE3), Na+/K+/2Cl- cotransporter (NKCC2), NaCl cotransporter (NCC), phosphorylated NCC (p-NCC), epithelial Na+ channel β (ENaCβ), pendrin, renal outer medullary K1 channel (ROMK), and large-conductance, voltage-activated and Ca2+-sensitive K+ channel (Maxi-K) were examined by immunoblotting of uEVs and immunofluorescence of biopsied kidney tissues. Healthy and disease (bulimic patients) controls were also enrolled. Results: Characterization of uEVs was confirmed by nanoparticle tracking analysis, transmission electron microscopy, and immunoblotting. Compared with healthy controls, uEVs from GS patients showed NCC and p-NCC abundance were markedly attenuated but NHE3, ENaCβ, and pendrin abundance significantly increased. ROMK and Maxi-K abundance were also significantly accentuated. Immunofluorescence of the representative kidney tissues from GS patients also demonstrated the similar findings to uEVs. uEVs from bulimic patients showed an increased abundance of NCC and p-NCC as well as NHE3, NKCC2, ENaCβ, pendrin, ROMK and Maxi-K, akin to that in immunofluorescence of their kidney tissues. Conclusion: uEVs could be a non-invasive tool to diagnose and evaluate renal tubular transporter adaptation in patients with GS and may be applied to other renal tubular diseases.
Keywords: Gitelman syndrome; hypokalemia; renal tubular disease; renal tubular transporters; urinary extracellular vesicles (exosomes).
Copyright © 2021 Sung, Chen, Lin, Lin, Lin, Yang and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

