Thermodynamic Analysis for Binding of 4- O-β-tri- N-acetylchitotriosyl Moranoline, a Transition State Analogue Inhibitor for Hen Egg White Lysozyme
- PMID: 34179076
- PMCID: PMC8222817
- DOI: 10.3389/fmolb.2021.654706
Thermodynamic Analysis for Binding of 4- O-β-tri- N-acetylchitotriosyl Moranoline, a Transition State Analogue Inhibitor for Hen Egg White Lysozyme
Abstract
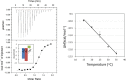
4-O-β-tri-N-acetylchitotriosyl moranoline (GN3M) is a transition-state analogue for hen egg white lysozyme (HEWL) and identified as the most potent inhibitor till date. Isothermal titration calorimetry experiments provided the thermodynamic parameters for binding of GN3M to HEWL and revealed that the binding is driven by a favorable enthalpy change (ΔH° = -11.0 kcal/mol) with an entropic penalty (-TΔS° = 2.6 kcal/mol), resulting in a free energy change (ΔG°) of -8.4 kcal/mol [Ogata et al. (2013) 288, 6,072-6,082]. Dissection of the entropic term showed that a favorable solvation entropy change (-TΔS solv° = -9.2 kcal/mol) is its sole contributor. The change in heat capacity (ΔC p°) for the binding of GN3M was determined to be -120.2 cal/K·mol. These results indicate that the bound water molecules play a crucial role in the tight interaction between GN3M and HEWL.
Keywords: 4-O-β-tri-N-acetylchitotriosyl moranoline; binding; inhibitor; lysozyme (HEWL); thermodynamics.
Copyright © 2021 Ogata, Fukamizo and Ohnuma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Arai M., Sumida M., Fukuhara K., Kainosho M., Murao S. (1986). Isolation and Characterization of Amylase Inhibitors, (Glucose)n·Deoxynojirimycin. Agric. Biol. Chem. 50, 639–644. 10.1080/00021369.1986.10867440 - DOI
LinkOut - more resources
Full Text Sources

