Weight Change and Predictors of Weight Change Among Patients Initiated on Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide or Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Real-World Retrospective Study
- PMID: 34179212
- PMCID: PMC8203466
- DOI: 10.36469/001c.24535
Weight Change and Predictors of Weight Change Among Patients Initiated on Darunavir/Cobicistat/Emtricitabine/Tenofovir Alafenamide or Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Real-World Retrospective Study
Abstract
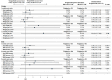
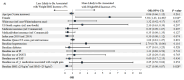
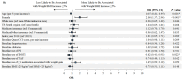
Background: Recent evidence suggests that integrase strand transfer inhibitors are associated with greater weight gain than protease inhibitors in patients with human immunodeficiency virus (HIV-1). Objectives: To describe demographic and clinical characteristics of insured patients with HIV-1 in the United States initiating darunavir/cobicistat/emtricitabine/tenofovir alafenamide (DRV/c/FTC/TAF) or bictegravir/FTC/TAF (BIC/FTC/TAF), assess the differences in weight and body mass index (BMI) change between cohorts up to one year after treatment initiation, and identify the predictors of weight gain associated with each treatment. Methods: The Symphony Health, IDV® database (July 17, 2017 - September 30, 2019) was used to identify treatment naïve or virologically suppressed stable switchers who initiated DRV/c/FTC/TAF or BIC/FTC/TAF (index date) on or after July 17, 2018, were ≥18 years of age on the index date, and had ≥12 months of continuous clinical activity pre-index (baseline period). To account for differences in baseline characteristics, inverse-probability of treatment weighting (IPTW) was used. Mean weight and BMI change from pre- to post-index measurements were compared between weighted cohorts at 3, 6, 9, and 12 months post-index using mean differences. Predictors of weight or BMI gain ≥5% were evaluated at last measurement, for each treatment cohort separately. Results: After IPTW, 452 and 497 patients were included in the DRV/c/FTC/TAF and BIC/FTC/TAF cohorts, respectively. Baseline characteristics were generally well-balanced (mean age=~50 years, female: ~30%), except for the type of antiretroviral therapy from which patients switched. Patients initiated on BIC/FTC/TAF experienced greater weight and BMI increases between the pre-index period and each measurement of the post-index period than patients initiated on DRV/c/FTC/TAF, although results were only statistically significant at 9 months post-index (weight: mean difference=2.50 kg, P=0.005; BMI: mean difference=0.66 kg/m2, P=0.027). A common predictor of weight or BMI gain ≥5% among patients in both cohorts was female gender (DRV/c/FTC/TAF: odds ratio [OR]=5.92, P=0.014; BIC/FTC/TAF: OR=2.00, P<0.001). Conclusion: Patients in the BIC/FTC/TAF cohort experienced greater weight and BMI increases than patients in the DRV/c/FTC/TAF cohort, with differences reaching statistical significance at 9 months post-index. Weight gain is an important factor to consider when selecting antiretroviral regimens, since it is associated with long-term health consequences. Future studies with larger sample size and longer follow-up time are warranted.
Keywords: body mass index; electronic health records; hiv; integrase inhibitors; observational study; protease inhibitors; weight gain.
Figures


References
-
- Viral suppression and HIV transmission in serodiscordant male couples: An international, prospective, observational, cohort study. Bavinton Benjamin R, Pinto Angie N, Phanuphak Nittaya, Grinsztejn Beatriz, Prestage Garrett P, Zablotska-Manos Iryna B, Jin Fengyi, Fairley Christopher K, Moore Richard, Roth Norman, Bloch Mark, Pell Catherine, McNulty Anna M, Baker David, Hoy Jennifer, Tee Ban Kiem, Templeton David J, Cooper David A, Emery Sean, Kelleher Anthony, Grulich Andrew E, Grulich Andrew E, Zablotska-Manos Iryna B, Prestage Garrett P, Jin Fengyi, Bavinton Benjamin R, Grinsztejn Beatriz, Phanuphak Nittaya, Cooper David A, Kelleher Anthony, Emery Sean, Fairley Christopher K, Wilson David, Koelsch Kersten K, Triffitt Kathy, Doong Nicolas, Baker David, Bloch Mark, Templeton David J, McNulty Anna, Pell Catherine, Hoy Jennifer, Tee Ban Kiem, Moore Richard, Roth Norman, Orth David, Pinto Angie N. Aug;2018 The Lancet HIV. 5(8):e438–e447. doi: 10.1016/s2352-3018(18)30132-2. doi: 10.1016/s2352-3018(18)30132-2. - DOI - DOI - PubMed
-
- Cohen Myron S., Chen Ying Q., McCauley Marybeth, Gamble Theresa, Hosseinipour Mina C., Kumarasamy Nagalingeswaran, Hakim James G., Kumwenda Johnstone, Grinsztejn Beatriz, Pilotto Jose H.S., Godbole Sheela V., Mehendale Sanjay, Chariyalertsak Suwat, Santos Breno R., Mayer Kenneth H., Hoffman Irving F., Eshleman Susan H., Piwowar-Manning Estelle, Wang Lei, Makhema Joseph, Mills Lisa A., de Bruyn Guy, Sanne Ian, Eron Joseph, Gallant Joel, Havlir Diane, Swindells Susan, Ribaudo Heather, Elharrar Vanessa, Burns David, Taha Taha E., Nielsen-Saines Karin, Celentano David, Essex Max, Fleming Thomas R. New England Journal of Medicine. 6. Vol. 365. Massachusetts Medical Society; Prevention of HIV-1 infection with early antiretroviral therapy; pp. 493–505. - DOI - DOI - PMC - PubMed
-
- Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: Late versus early diagnosis and entry into care. Farnham Paul G., Gopalappa Chaitra, Sansom Stephanie L., Hutchinson Angela B., Brooks John T., Weidle Paul J., Marconi Vincent C., Rimland David. Oct 1;2013 J Acquir Immune Defic Syndr. 64(2):183–189. doi: 10.1097/qai.0b013e3182973966. doi: 10.1097/qai.0b013e3182973966. - DOI - DOI - PubMed
-
- Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. Quinn Thomas C., Wawer Maria J., Sewankambo Nelson, Serwadda David, Li Chuanjun, Wabwire-Mangen Fred, Meehan Mary O., Lutalo Thomas, Gray Ronald H. Mar 30;2000 New England Journal of Medicine. 342(13):921–929. doi: 10.1056/nejm200003303421303. doi: 10.1056/nejm200003303421303. - DOI - DOI - PubMed
LinkOut - more resources
Full Text Sources
