Bone morphogenetic protein 4 (BMP4) promotes hepatic glycogen accumulation and reduces glucose level in hepatocytes through mTORC2 signaling pathway
- PMID: 34179315
- PMCID: PMC8209350
- DOI: 10.1016/j.gendis.2020.11.004
Bone morphogenetic protein 4 (BMP4) promotes hepatic glycogen accumulation and reduces glucose level in hepatocytes through mTORC2 signaling pathway
Abstract
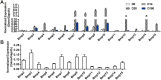
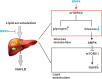
Liver is an important organ for regulating glucose and lipid metabolism. Recent studies have shown that bone morphogenetic proteins (BMPs) may play important roles in regulating glucose and lipid metabolism. In our previous studies, we demonstrated that BMP4 significantly inhibits hepatic steatosis and lowers serum triglycerides, playing a protective role against the progression of non-alcoholic fatty liver disease (NAFLD). However, the direct impact of BMP4 on hepatic glucose metabolism is poorly understood. Here, we investigated the regulatory roles of BMP4 in hepatic glucose metabolism. Through a comprehensive analysis of the 14 types of BMPs, we found that BMP4 was one of the most potent BMPs in promoting hepatic glycogen accumulation, reducing the level of glucose in hepatocytes and effecting the expression of genes related to glucose metabolism. Mechanistically, we demonstrated that BMP4 reduced the hepatic glucose levels through the activation of mTORC2 signaling pathway in vitro and in vivo. Collectively, our findings strongly suggest that BMP4 may play an essential role in regulating hepatic glucose metabolism. This knowledge should aid us to understand the molecular pathogenesis of NAFLD, and may lead to the development of novel therapeutics by exploiting the inhibitory effects of BMPs on hepatic glucose and lipid metabolism.
Keywords: BMP4; Glucose metabolism; Glycogen accumulation; Non-alcoholic fatty liver disease (NAFLD); mTORC2 signaling.
© 2020 Chongqing Medical University. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare they have no competing interesting.
Figures






Similar articles
-
Bone morphogenetic protein 4 (BMP4) alleviates hepatic steatosis by increasing hepatic lipid turnover and inhibiting the mTORC1 signaling axis in hepatocytes.Aging (Albany NY). 2019 Dec 12;11(23):11520-11540. doi: 10.18632/aging.102552. Epub 2019 Dec 12. Aging (Albany NY). 2019. PMID: 31831718 Free PMC article.
-
Knockdown of long non-coding RNA Gm10804 suppresses disorders of hepatic glucose and lipid metabolism in diabetes with non-alcoholic fatty liver disease.Cell Biochem Funct. 2020 Oct;38(7):839-846. doi: 10.1002/cbf.3495. Epub 2020 Mar 24. Cell Biochem Funct. 2020. PMID: 32212193
-
Thrombospondin 1 improves hepatic steatosis in diet-induced insulin-resistant mice and is associated with hepatic fat content in humans.EBioMedicine. 2020 Jul;57:102849. doi: 10.1016/j.ebiom.2020.102849. Epub 2020 Jun 21. EBioMedicine. 2020. PMID: 32580141 Free PMC article.
-
Emerging Role of Bone Morphogenetic Protein 4 in Metabolic Disorders.Diabetes. 2021 Feb;70(2):303-312. doi: 10.2337/db20-0884. Diabetes. 2021. PMID: 33472940 Review.
-
The link between Hepatic Vitamin A Metabolism and Nonalcoholic Fatty Liver Disease.Curr Drug Targets. 2015;16(12):1281-92. doi: 10.2174/1389450116666150325231015. Curr Drug Targets. 2015. PMID: 25808650 Review.
Cited by
-
Bone morphogenetic protein 4 alleviates nonalcoholic steatohepatitis by inhibiting hepatic ferroptosis.Cell Death Discov. 2022 Apr 27;8(1):234. doi: 10.1038/s41420-022-01011-7. Cell Death Discov. 2022. PMID: 35477568 Free PMC article.
-
OUHP: an optimized universal hairpin primer system for cost-effective and high-throughput RT-qPCR-based quantification of microRNA (miRNA) expression.Nucleic Acids Res. 2022 Feb 28;50(4):e22. doi: 10.1093/nar/gkab1153. Nucleic Acids Res. 2022. PMID: 34850128 Free PMC article.
-
Examining the Pathogenesis of MAFLD and the Medicinal Properties of Natural Products from a Metabolic Perspective.Metabolites. 2024 Apr 12;14(4):218. doi: 10.3390/metabo14040218. Metabolites. 2024. PMID: 38668346 Free PMC article. Review.
-
Niclosamide (NA) overcomes cisplatin resistance in human ovarian cancer.Genes Dis. 2023 Jan 2;10(4):1687-1701. doi: 10.1016/j.gendis.2022.12.005. eCollection 2023 Jul. Genes Dis. 2023. PMID: 37397523 Free PMC article.
-
Expression of BMP-4 in dentigerous cyst and ameloblastoma: Is it a differentiation measure?Eur Oral Res. 2022 Jan 1;56(1):17-22. doi: 10.26650/eor.2022903844. Eur Oral Res. 2022. PMID: 35478708 Free PMC article.
References
-
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. - PubMed
-
- Younossi Z.M., Marchesini G., Pinto-Cortez H., Petta S. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: implications for liver transplantation. Transplantation. 2019;103(1):22–27. - PubMed
-
- Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–S64. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

