Vaccine induced thrombotic thrombocytopenia: The shady chapter of a success story
- PMID: 34179744
- PMCID: PMC8217988
- DOI: 10.1016/j.metop.2021.100101
Vaccine induced thrombotic thrombocytopenia: The shady chapter of a success story
Abstract
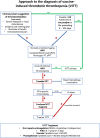
The recognition of the rare but serious and potentially lethal complication of vaccine induced thrombotic thrombocytopenia (VITT) raised concerns regarding the safety of COVID-19 vaccines and led to the reconsideration of vaccination strategies in many countries. Following the description of VITT among recipients of adenoviral vector ChAdOx1 vaccine, a review of similar cases after Ad26.COV2·S vaccination gave rise to the question whether this entity may constitute a potential class effect of all adenoviral vector vaccines. Most cases are females, typically younger than 60 years who present shortly (range: 5-30 days) following vaccination with thrombocytopenia and thrombotic manifestations, occasionally in multiple sites. Following initial incertitude, concrete recommendations to guide the diagnosis (clinical suspicion, initial laboratory screening, PF4-polyanion-antibody ELISA) and management of VITT (non-heparin anticoagulants, corticosteroids, intravenous immunoglobulin) have been issued. The mechanisms behind this rare syndrome are currently a subject of active research and include the following: 1) production of PF4-polyanion autoantibodies; 2) adenoviral vector entry in megacaryocytes and subsequent expression of spike protein on platelet surface; 3) direct platelet and endothelial cell binding and activation by the adenoviral vector; 4) activation of endothelial and inflammatory cells by the PF4-polyanion autoantibodies; 5) the presence of an inflammatory co-signal; and 6) the abundance of circulating soluble spike protein variants following vaccination. Apart from the analysis of potential underlying mechanisms, this review aims to synopsize the clinical and epidemiologic features of VITT, to present the current evidence-based recommendations on diagnostic and therapeutic work-up of VITT and to discuss new dilemmas and perspectives that emerged after the description of this entity.
Keywords: Adenoviral vector; Adenovirus; CAR, Coxsackie-adenovirus receptor; CDC, Centers for Disease Control and Prevention; COVID-19; COVID-19, Coronavirus disease 2019; CVST, cerebellar sinus thrombosis; FDA, Food and Drug Administration; HIT, Heparin-induced thrombocytopenia; ICU, Intensive Care Unit; IVIG, Intravenous immunoglobulin; LMWH, low molecular weight heparin; PF4, Platelet factor 4; PLT, Platelet; PRAC, Pharmacovigilance Risk Assessment Committee; PT, prothrombin time; SARS-CoV-2; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2; SVT, splanchnic vein thrombosis; TTS, thrombosis-thrombocytopenia-syndrome; VCAM-1, vascular cell adhesion molecule 1; VIPIT, vaccine-induced prothrombotic immune thrombocytopenia; VITT, vaccine induced thrombotic thrombocytopenia; Vaccine; Vaccine induced thrombotic thrombocytopenia; aPTT, activated partial thromboplastin time; ΕΜΑ, European Medicines Agency.
© 2021 Published by Elsevier Inc.
Conflict of interest statement
No conflict of interest to disclose
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous