Comparison of effect of crush or transection peripheral nerve lesion on lumbar spinal cord synaptic plasticity and microglial dynamics
- PMID: 34179871
- PMCID: PMC8211924
- DOI: 10.1016/j.ibneur.2021.05.002
Comparison of effect of crush or transection peripheral nerve lesion on lumbar spinal cord synaptic plasticity and microglial dynamics
Abstract
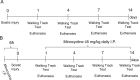
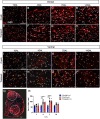
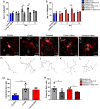
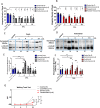
In an injury to the peripheral nervous system, the spinal cord and brain structure reorganize connections to optimize the function of the remaining parts. Many cell events are triggered in the spinal cord to support changes in the synaptic connections around motoneurons, where old connections are removed, and new ones created. Microglial cells are primitive macrophages that invade the central nervous system in early stages of neurodevelopment and have several functions, such as eliminating synapses. We investigated the synaptic plasticity after different types of peripheral (sciatic) nerve injury (crush or total transection), as well as the behavior of microglial cells for 2 weeks after a peripheral lesion. As expected, sciatic-nerve injury reduced motor performance in mice, but crushed animals regained partial motor control. Because of sciatic-nerve injury, pre-synaptic inputs decreased around the motoneurons in the ventro-lateral horn, while microglial cells increased around these cells. Microglial cells also exhibited altered morphology in both types of peripheral lesion, indicating a similar underlying mechanism of plasticity. To investigate the involvement of microglia in this scenario, microglial activation was modulated by daily administration of minocycline. The minocycline treatment directly affected the microglial response and impacted the synapse rearrangement in the spinal cord. Together, these results demonstrate that microglia cells are involved in synaptic plasticity in the lumbar spinal cord in both nerve-injury scenarios.
Summary of statement: Here, we demonstrated that acute plasticity in the lumbar spinal cord (LSC) did not differ between crush and transection of peripheral nerve, and that microglial reactivity in the LSC was important after both injury types.
Keywords: Lumbar spinal cord; Microglia; Sciatic nerve; Synaptic plasticity.
© 2021 The Authors.
Figures

References
-
- Aldskogius H. Mechanisms and consequences of microglial responses to peripheral axotomy. Front. Biosci. 2011;3:857–868. - PubMed
-
- Aldskogius H., Kozlova E.N. Central neuron-glial and glial-glial interactions following axon injury. Prog. Neurobiol. 1998;55(1):1–26. - PubMed
-
- Arbat-Plana A., Torres-Espín A., Navarro X., Udina E. Activity dependent therapies modulate the spinal changes that motoneurons suffer after a peripheral nerve injury. Exp. Neurol. 2015;263:293–305. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
