Synthesis of pH Dependent Pyrazole, Imidazole, and Isoindolone Dipyrrinone Fluorophores using a Claisen-Schmidt Condensation Approach
- PMID: 34180889
- PMCID: PMC8498924
- DOI: 10.3791/61944
Synthesis of pH Dependent Pyrazole, Imidazole, and Isoindolone Dipyrrinone Fluorophores using a Claisen-Schmidt Condensation Approach
Abstract
Methine-bridged conjugated bicyclic aromatic compounds are common constituents of a range of biologically relevant molecules such as porphyrins, dipyrrinones, and pharmaceuticals. Additionally, restricted rotation of these systems often results in highly to moderately fluorescent systems as observed in 3H,5H-dipyrrolo[1,2-c:2',1'-f]pyrimidin-3-ones, xanthoglows, pyrroloindolizinedione analogs, BODIPY analogs, and the phenolic and imidazolinone ring systems of Green Fluorescent Protein (GFP). This manuscript describes an inexpensive and operationally simple method of performing a Claisen-Schmidt condensation to generate a series of fluorescent pH dependent pyrazole/imidazole/isoindolone dipyrrinone analogs. While the methodology illustrates the synthesis of dipyrrinone analogs, it can be translated to produce a wide range of conjugated bicyclic aromatic compounds. The Claisen-Schmidt condensation reaction utilized in this method is limited in scope to nucleophiles and electrophiles that are enolizable under basic conditions (nucleophile component) and non-enolizable aldehydes (electrophile component). Additionally, both the nucleophilic and electrophilic reactants must contain functional groups that will not inadvertently react with hydroxide. Despite these limitations, this methodology offers access to completely novel systems that can be employed as biological or molecular probes.
Figures


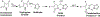

References
-
- Abbandonato G; Signore G; Nifosi R; Voliani V; Bizzarri R; Beltram F, Cis-trans photoisomerization properties of GFP chromophore analogs. European Biophysics Journal 2011, 40 (11), 1205–1214. - PubMed
-
- Funakoshi H; Haba K; Kobara K; Taniguchi H; Tamagake K; Fujita Y, Spectroscopic studies on merocyanine photoisomers. IV. Catalytic isomerization of photoisomers of merocyanine derivatives in protic solvents. Nippon Kagaku Kaishi 1989, (9), 1516–22.
-
- Puzicha G; Shrout DP; Lightner DA, Synthesis and properties of homomologated and contracted dipyrrinone analogs of xanthobilirubic acid. Journal of Heterocyclic Chemistry 1990, 27 (7), 2117–23.
-
- Bonnett R; Hamzetash D; Asuncion Valles M, Propentdyopents [5-(2-oxo-2H-pyrrol-5-ylmethylene)pyrrol-2(5H)-ones] and related compounds. Part 2. The Z ↹ E photoisomerization of pyrromethenone systems. Journal of the Chemical Society, Perkins Transactions 1 1987, (6), 1383–8.
-
- Tikhomirova K; Anisimov A; Khoroshutin A, Biscyclohexane-Annulated Diethyl Dipyrrindicarboxylates: Observation of a Dipyrrin Form with Absent Visible Absorption. European Journal of Organic Chemistry 2012, 2012 (11), 2201–2207, S2201/1–S2201/8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
