A Systematic Review and Meta-Analysis on the Efficacy of Curcumin/Turmeric for the Prevention and Amelioration of Radiotherapy/Radiochemotherapy Induced Oral Mucositis in Head and Neck Cancer Patients
- PMID: 34181321
- PMCID: PMC8418840
- DOI: 10.31557/APJCP.2021.22.6.1671
A Systematic Review and Meta-Analysis on the Efficacy of Curcumin/Turmeric for the Prevention and Amelioration of Radiotherapy/Radiochemotherapy Induced Oral Mucositis in Head and Neck Cancer Patients
Abstract
Background: Oral Mucositis(OM) is an acute debilitating dose limiting toxicity of Radiotherapy/Radiochemotherapy(RT/RCT) in management of Head and Neck Cancer (HNC). Curcumin/Turmeric may reduce OM in patients.
Aim: Efficacy of Curcumin/Turmeric for preventing and ameliorating the onset and severity of RT/RCT induced OM was analysed in this review.
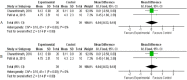
Methods: A systematic literature search with meta-analysis were performed using Mesh terms in PubMed, Google scholar, Science Direct, Cochrane library and manual searching, articles published from 2010 to April 2021 were included. Clinical trials that studied the efficacy/effects of turmeric / curcumin in management of RT/RCT induced OM in HNC patients were included. Statistical Analysis were done to calculate the pooled Risk ratio at 95%confidence interval with significance at p.
Keywords: Oral Mucositis; Turmeric; curcumin; head and neck cancer; radiotherapy.
Figures








References
-
- Adhvaryu M, Vakharia B, Reddy N. Curcumin Prevents Mucositis and Improves Patient Compliance in Head & Neck Cancer Patients Undergoing Radio-Chemotherapy. Ann Med Chem Res. 2018;4:1022.
-
- Arun P, Sagayaraj A, Mohiyuddin SA, et al. Role of turmeric extract in minimising mucositis in patients receiving radiotherapy for head and neck squamous cell cancer: a randomised, placebo-controlled trial. J Laryngol Otol. 2020;134:159–4. - PubMed
-
- Bensinger W, Schubert M, Ang KK, et al. NCCN task force report: prevention and management of mucositis in cancer care. J Nat Comprehensive Cancer Network. 2008;6:S–1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

