Oxytocin levels in response to pituitary provocation tests in healthy volunteers
- PMID: 34181566
- PMCID: PMC8650762
- DOI: 10.1530/EJE-21-0346
Oxytocin levels in response to pituitary provocation tests in healthy volunteers
Abstract
Objective: Oxytocin, secreted into circulation through the posterior pituitary, regulates lactation, weight, and socio-behavioral functioning. Oxytocin deficiency has been suggested in patients with hypopituitarism; however, diagnostic testing for oxytocin deficiency has not been developed. The aim of this study was to investigate known pituitary provocation tests to stimulate plasma oxytocin.
Design: Sixty-five healthy volunteers underwent either the hypertonic saline or arginine infusion test, known to stimulate copeptin, or the oral macimorelin test, known to stimulate growth hormone. Plasma oxytocin was measured before and once plasma sodium level ≥ 150 mmol/L for the hypertonic saline, after 60 min for the arginine infusion, and after 45 min for the oral macimorelin test (expected peak of copeptin and growth hormone levels, respectively). Primary outcome was a change from basal to stimulated oxytocin levels using paired t-tests.
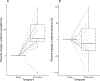
Results: As expected, copeptin increased in response to hypertonic saline and arginine infusion (P < 0.001), and growth hormone increased to oral macimorelin (P < 0.001). Oxytocin increased in response to hypertonic saline infusion from 0.4 (0.2) to 0.6 pg/mL (0.3) (P = 0.003) but with a high variance. There was no change to arginine infusion (P = 0.4), and a trend to lower stimulated levels to oral macimorelin (P = 0.05).
Conclusion: Neither the arginine infusion nor the oral macimorelin test stimulates plasma oxytocin levels, whereas there was an increase with high variance upon hypertonic saline infusion. As a predictable rise in most participants is required for a reliable pituitary provocation test, none of the investigated pituitary provocation tests can be recommended diagnostically to identify patients with an oxytocin deficiency.
Figures

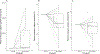
References
-
- Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides [Internet]. 1989. [cited 2020 Sep 8];10(1):89–93. Available from: https://pubmed.ncbi.nlm.nih.gov/2748428/ - PubMed
-
- Herisson FM, Waas JR, Fredriksson R, Schiöth HB, Levine AS, Olszewski PK. Oxytocin Acting in the Nucleus Accumbens Core Decreases Food Intake. J Neuroendocrinol [Internet]. 2016. April 1 [cited 2021 Jan 26];28(4). Available from: https://pubmed.ncbi.nlm.nih.gov/27114001/ - PubMed
-
- Tamma R, Colaianni G, Zhu L, DiBenedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, et al. Oxytocin is an anabolic bone hormone. Natl Acad Sci [Internet]. [cited 2019 Aug 9]; Available from: https://www.pnas.org/content/106/17/7149/ - PMC - PubMed
-
- Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Maréchal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology [Internet]. 2007. May [cited 2019 Aug 9];32(4):407–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0306453007000297 - PubMed
-
- Hoge EA, Lawson EA, Metcalf CA, Keshaviah A, Zak PJ, Pollack MH, Simon NM. Plasma oxytocin immunoreactive products and response to trust in patients with social anxiety disorder. Depress Anxiety [Internet]. 2012. November [cited 2019 Aug 9];29(11):924–30. Available from: 10.1002/da.21973 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

