Evolution of plant telomerase RNAs: farther to the past, deeper to the roots
- PMID: 34181710
- PMCID: PMC8287931
- DOI: 10.1093/nar/gkab545
Evolution of plant telomerase RNAs: farther to the past, deeper to the roots
Abstract
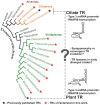
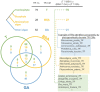
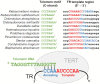
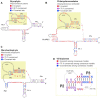
The enormous sequence heterogeneity of telomerase RNA (TR) subunits has thus far complicated their characterization in a wider phylogenetic range. Our recent finding that land plant TRs are, similarly to known ciliate TRs, transcribed by RNA polymerase III and under the control of the type-3 promoter, allowed us to design a novel strategy to characterize TRs in early diverging Viridiplantae taxa, as well as in ciliates and other Diaphoretickes lineages. Starting with the characterization of the upstream sequence element of the type 3 promoter that is conserved in a number of small nuclear RNAs, and the expected minimum TR template region as search features, we identified candidate TRs in selected Diaphoretickes genomes. Homologous TRs were then used to build covariance models to identify TRs in more distant species. Transcripts of the identified TRs were confirmed by transcriptomic data, RT-PCR and Northern hybridization. A templating role for one of our candidates was validated in Physcomitrium patens. Analysis of secondary structure demonstrated a deep conservation of motifs (pseudoknot and template boundary element) observed in all published TRs. These results elucidate the evolution of the earliest eukaryotic TRs, linking the common origin of TRs across Diaphoretickes, and underlying evolutionary transitions in telomere repeats.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






Similar articles
-
The conserved structure of plant telomerase RNA provides the missing link for an evolutionary pathway from ciliates to humans.Proc Natl Acad Sci U S A. 2019 Dec 3;116(49):24542-24550. doi: 10.1073/pnas.1915312116. Epub 2019 Nov 21. Proc Natl Acad Sci U S A. 2019. PMID: 31754031 Free PMC article.
-
Telomerase RNA in Hymenoptera (Insecta) switched to plant/ciliate-like biogenesis.Nucleic Acids Res. 2023 Jan 11;51(1):420-433. doi: 10.1093/nar/gkac1202. Nucleic Acids Res. 2023. PMID: 36546771 Free PMC article.
-
Telomerase RNA evolution: a journey from plant telomeres to broader eukaryotic diversity.Biochem J. 2025 Jan 31;482(3):169-79. doi: 10.1042/BCJ20240501. Biochem J. 2025. PMID: 39889303 Free PMC article. Review.
-
Telomerase RNAs in land plants.Nucleic Acids Res. 2019 Oct 10;47(18):9842-9856. doi: 10.1093/nar/gkz695. Nucleic Acids Res. 2019. PMID: 31392988 Free PMC article.
-
Telomerase RNA is more than a DNA template.RNA Biol. 2016 Aug 2;13(8):683-9. doi: 10.1080/15476286.2016.1191725. Epub 2016 May 31. RNA Biol. 2016. PMID: 27245259 Free PMC article. Review.
Cited by
-
Plant telomere biology: The green solution to the end-replication problem.Plant Cell. 2022 Jul 4;34(7):2492-2504. doi: 10.1093/plcell/koac122. Plant Cell. 2022. PMID: 35511166 Free PMC article.
-
GERONIMO: A tool for systematic retrieval of structural RNAs in a broad evolutionary context.Gigascience. 2022 Dec 28;12:giad080. doi: 10.1093/gigascience/giad080. Epub 2023 Oct 17. Gigascience. 2022. PMID: 37848616 Free PMC article.
-
Editorial: Telomere Flexibility and Versatility: A Role of Telomeres in Adaptive Potential.Front Genet. 2021 Oct 4;12:771938. doi: 10.3389/fgene.2021.771938. eCollection 2021. Front Genet. 2021. PMID: 34671387 Free PMC article. No abstract available.
-
Characterisation of the Arabidopsis thaliana telomerase TERT-TR complex.Plant Mol Biol. 2024 May 14;114(3):56. doi: 10.1007/s11103-024-01461-w. Plant Mol Biol. 2024. PMID: 38743198 Free PMC article.
-
Monkeyflower (Mimulus) uncovers the evolutionary basis of the eukaryote telomere sequence variation.PLoS Genet. 2025 Jun 16;21(6):e1011738. doi: 10.1371/journal.pgen.1011738. eCollection 2025 Jun. PLoS Genet. 2025. PMID: 40523014 Free PMC article.
References
-
- Sykorova E., Fajkus J.. Structure-function relationships in telomerase genes. Biol. Cell. 2009; 101:375–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

