Assembly of higher-order SMN oligomers is essential for metazoan viability and requires an exposed structural motif present in the YG zipper dimer
- PMID: 34181727
- PMCID: PMC8287954
- DOI: 10.1093/nar/gkab508
Assembly of higher-order SMN oligomers is essential for metazoan viability and requires an exposed structural motif present in the YG zipper dimer
Abstract
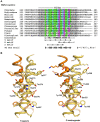
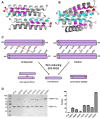
Protein oligomerization is one mechanism by which homogenous solutions can separate into distinct liquid phases, enabling assembly of membraneless organelles. Survival Motor Neuron (SMN) is the eponymous component of a large macromolecular complex that chaperones biogenesis of eukaryotic ribonucleoproteins and localizes to distinct membraneless organelles in both the nucleus and cytoplasm. SMN forms the oligomeric core of this complex, and missense mutations within its YG box domain are known to cause Spinal Muscular Atrophy (SMA). The SMN YG box utilizes a unique variant of the glycine zipper motif to form dimers, but the mechanism of higher-order oligomerization remains unknown. Here, we use a combination of molecular genetic, phylogenetic, biophysical, biochemical and computational approaches to show that formation of higher-order SMN oligomers depends on a set of YG box residues that are not involved in dimerization. Mutation of key residues within this new structural motif restricts assembly of SMN to dimers and causes locomotor dysfunction and viability defects in animal models.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Liu H., Cao M., Wang Y., Lv B., Li C.. Bioengineering oligomerization and monomerization of enzymes: learning from natural evolution to matching the demands for industrial applications. Crit. Rev. Biotechnol. 2020; 40:231–246. - PubMed
-
- Ali M.H., Imperiali B.. Protein oligomerization: how and why. Bioorg. Med. Chem. 2005; 13:5013–5020. - PubMed
-
- Gruss O.J., Meduri R., Schilling M., Fischer U.. UsnRNP biogenesis: mechanisms and regulation. Chromosoma. 2017; 126:577–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

