Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial
- PMID: 34181880
- PMCID: PMC8233007
- DOI: 10.1016/S0140-6736(21)01420-3
Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial
Erratum in
-
Department of Error.Lancet. 2021 Aug 14;398(10300):582. doi: 10.1016/S0140-6736(21)01805-5. Lancet. 2021. PMID: 34391500 Free PMC article. No abstract available.
Abstract
Background: To date, no immunological data on COVID-19 heterologous vaccination schedules in humans have been reported. We assessed the immunogenicity and reactogenicity of BNT162b2 (Comirnaty, BioNTech, Mainz, Germany) administered as second dose in participants primed with ChAdOx1-S (Vaxzevria, AstraZeneca, Oxford, UK).
Methods: We did a phase 2, open-label, randomised, controlled trial on adults aged 18-60 years, vaccinated with a single dose of ChAdOx1-S 8-12 weeks before screening, and no history of SARS-CoV-2 infection. Participants were randomly assigned (2:1) to receive either BNT162b2 (0·3 mL) via a single intramuscular injection (intervention group) or continue observation (control group). The primary outcome was 14-day immunogenicity, measured by immunoassays for SARS-CoV-2 trimeric spike protein and receptor binding domain (RBD). Antibody functionality was assessed using a pseudovirus neutralisation assay, and cellular immune response using an interferon-γ immunoassay. The safety outcome was 7-day reactogenicity, measured as solicited local and systemic adverse events. The primary analysis included all participants who received at least one dose of BNT162b2 and who had at least one efficacy evaluation after baseline. The safety analysis included all participants who received BNT162b2. This study is registered with EudraCT (2021-001978-37) and ClinicalTrials.gov (NCT04860739), and is ongoing.
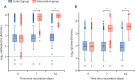
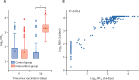
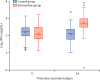
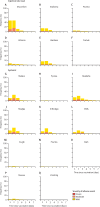
Findings: Between April 24 and 30, 2021, 676 individuals were enrolled and randomly assigned to either the intervention group (n=450) or control group (n=226) at five university hospitals in Spain (mean age 44 years [SD 9]; 382 [57%] women and 294 [43%] men). 663 (98%) participants (n=441 intervention, n=222 control) completed the study up to day 14. In the intervention group, geometric mean titres of RBD antibodies increased from 71·46 BAU/mL (95% CI 59·84-85·33) at baseline to 7756·68 BAU/mL (7371·53-8161·96) at day 14 (p<0·0001). IgG against trimeric spike protein increased from 98·40 BAU/mL (95% CI 85·69-112·99) to 3684·87 BAU/mL (3429·87-3958·83). The interventional:control ratio was 77·69 (95% CI 59·57-101·32) for RBD protein and 36·41 (29·31-45·23) for trimeric spike protein IgG. Reactions were mild (n=1210 [68%]) or moderate (n=530 [30%]), with injection site pain (n=395 [88%]), induration (n=159 [35%]), headache (n=199 [44%]), and myalgia (n=194 [43%]) the most commonly reported adverse events. No serious adverse events were reported.
Interpretation: BNT162b2 given as a second dose in individuals prime vaccinated with ChAdOx1-S induced a robust immune response, with an acceptable and manageable reactogenicity profile.
Funding: Instituto de Salud Carlos III.
Translations: For the French and Spanish translations of the abstract see Supplementary Materials section.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests CB-I is the deputy general manager of the Instituto de Salud Carlos III. JRA has received fees from Janssen, outside of the submitted work. AMB is principal investigator of clinical trials sponsored by GlaxoSmithKline, Daiichi-Sankyo, Janssen, and Farmalider, outside of the submitted work. All other authors declare no competing interests.
Figures
Comment in
-
Heterologous vaccine regimens against COVID-19.Lancet. 2021 Jul 10;398(10295):94-95. doi: 10.1016/S0140-6736(21)01442-2. Epub 2021 Jun 25. Lancet. 2021. PMID: 34181881 Free PMC article. No abstract available.
-
SARS-CoV-2 delta variant neutralisation after heterologous ChAdOx1-S/BNT162b2 vaccination.Lancet. 2021 Sep 18;398(10305):1041-1042. doi: 10.1016/S0140-6736(21)01891-2. Epub 2021 Aug 17. Lancet. 2021. PMID: 34416198 Free PMC article. No abstract available.
-
BioNTech/Pfizer vaccine after Oxford/AZ vaccine increased immune response vs. no second vaccine.Ann Intern Med. 2021 Nov;174(11):JC123. doi: 10.7326/ACPJ202111160-123. Epub 2021 Nov 2. Ann Intern Med. 2021. PMID: 34724407
References
-
- Kardani K, Bolhassani A, Shahbazi S. Prime-boost vaccine strategy against viral infections: mechanisms and benefits. Vaccine. 2016;34:413–423. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

