Human neural organoids: Models for developmental neurobiology and disease
- PMID: 34181916
- PMCID: PMC8364509
- DOI: 10.1016/j.ydbio.2021.06.012
Human neural organoids: Models for developmental neurobiology and disease
Abstract
Human organoids stand at the forefront of basic and translational research, providing experimentally tractable systems to study human development and disease. These stem cell-derived, in vitro cultures can generate a multitude of tissue and organ types, including distinct brain regions and sensory systems. Neural organoid systems have provided fundamental insights into molecular mechanisms governing cell fate specification and neural circuit assembly and serve as promising tools for drug discovery and understanding disease pathogenesis. In this review, we discuss several human neural organoid systems, how they are generated, advances in 3D imaging and bioengineering, and the impact of organoid studies on our understanding of the human nervous system.
Keywords: Brain; CRISPR; Cerebellar organoid; Cerebellum; Cerebral cortex; Cortical organoid; Embryoid bodies; Embryonic stem cells; Hippocampal organoid; Hippocampus; Human; Hypothalamic organoid; Hypothalamus; Induced-pluripotent stem cells; Organoid; Retina; Retinal organoid; Thalamic organoid; Thalamus; scRNA-seq.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
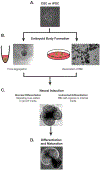
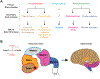
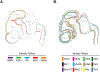

References
-
- Agirman G, Broix L, and Nguyen L (2017). Cerebral cortex development: an outside-in perspective. FEBS Lett 591, 3978–3992. - PubMed
-
- Allen I (2008). Allen Developing Mouse Brain Atlas.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

