Defective Flow-Migration Coupling Causes Arteriovenous Malformations in Hereditary Hemorrhagic Telangiectasia
- PMID: 34182767
- PMCID: PMC8429266
- DOI: 10.1161/CIRCULATIONAHA.120.053047
Defective Flow-Migration Coupling Causes Arteriovenous Malformations in Hereditary Hemorrhagic Telangiectasia
Abstract
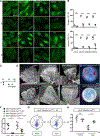
Background: Activin receptor-like kinase 1 (ALK1) is an endothelial transmembrane serine threonine kinase receptor for BMP family ligands that plays a critical role in cardiovascular development and pathology. Loss-of-function mutations in the ALK1 gene cause type 2 hereditary hemorrhagic telangiectasia, a devastating disorder that leads to arteriovenous malformations. Here, we show that ALK1 controls endothelial cell polarization against the direction of blood flow and flow-induced endothelial migration from veins through capillaries into arterioles.
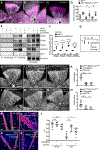
Methods: Using Cre lines that recombine in different subsets of arterial, capillary-venous, or endothelial tip cells, we show that capillary-venous Alk1 deletion was sufficient to induce arteriovenous malformation formation in the postnatal retina.
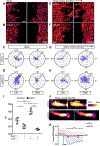
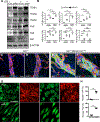
Results: ALK1 deletion impaired capillary-venous endothelial cell polarization against the direction of blood flow in vivo and in vitro. Mechanistically, ALK1-deficient cells exhibited increased integrin signaling interaction with vascular endothelial growth factor receptor 2, which enhanced downstream YAP/TAZ nuclear translocation. Pharmacologic inhibition of integrin or YAP/TAZ signaling rescued flow migration coupling and prevented vascular malformations in Alk1-deficient mice.
Conclusions: Our study reveals ALK1 as an essential driver of flow-induced endothelial cell migration and identifies loss of flow-migration coupling as a driver of arteriovenous malformation formation in hereditary hemorrhagic telangiectasia disease. Integrin-YAP/TAZ signaling blockers are new potential targets to prevent vascular malformations in patients with hereditary hemorrhagic telangiectasia.
Keywords: arteriovenous malformations; cell movement; telangiectasia, hereditary hemorrhagic; vascular endothelial growth factor A.
Figures



References
-
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrel J, et al.Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nature Genetics. 1994;8:345–351. doi: 10.1038/ng1294-345 - DOI - PubMed
-
- Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ and Marchuk DA. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet. 2004;363:852–9. doi: 10.1016/s0140-6736(04)15732-2 - DOI - PubMed
-
- Gallione CJ, Klaus DJ, Yeh EY, Stenzel TT, Xue Y, Anthony KB, McAllister KA, Baldwin MA, Berg JN, Lux A, et al.Mutation and expression analysis of the endoglin gene in Hereditary Hemorrhagic Telangiectasia reveals null alleles. Human Mutation. 1998;11:286–294. doi: 10.1002/(SICI)1098-1004(1998)11:4<286::AID-HUMU6>3.0.CO;2-B - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

