Protein Prenylation and Hsp40 in Thermotolerance of Plasmodium falciparum Malaria Parasites
- PMID: 34182772
- PMCID: PMC8262983
- DOI: 10.1128/mBio.00760-21
Protein Prenylation and Hsp40 in Thermotolerance of Plasmodium falciparum Malaria Parasites
Abstract
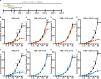
During its complex life cycle, the malaria parasite survives dramatic environmental stresses, including large temperature shifts. Protein prenylation is required during asexual replication of Plasmodium falciparum, and the canonical heat shock protein 40 protein (HSP40; PF3D7_1437900) is posttranslationally modified with a 15-carbon farnesyl isoprenyl group. In other organisms, farnesylation of Hsp40 orthologs controls their localization and function in resisting environmental stress. In this work, we find that plastidial isopentenyl pyrophosphate (IPP) synthesis and protein farnesylation are required for malaria parasite survival after cold and heat shock. Furthermore, loss of HSP40 farnesylation alters its membrane attachment and interaction with proteins in essential pathways in the parasite. Together, this work reveals that farnesylation is essential for parasite survival during temperature stress. Farnesylation of HSP40 may promote thermotolerance by guiding distinct chaperone-client protein interactions.
Keywords: Plasmodium falciparum; farnesylation; heat shock; isoprenoids; malaria; protein chaperone.
Figures







References
-
- Siau A, Silvie O, Franetich J-F, Yalaoui S, Marinach C, Hannoun L, van Gemert G-J, Luty AJF, Bischoff E, David PH, Snounou G, Vaquero C, Froissard P, Mazier D. 2008. Temperature shift and host cell contact up-regulate sporozoite expression of Plasmodium falciparum genes involved in hepatocyte infection. PLoS Pathog 4:e1000121. doi:10.1371/journal.ppat.1000121. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
