Bronchial thermoplasty in asthma: an exploratory histopathological evaluation in distinct asthma endotypes/phenotypes
- PMID: 34183014
- PMCID: PMC8240300
- DOI: 10.1186/s12931-021-01774-0
Bronchial thermoplasty in asthma: an exploratory histopathological evaluation in distinct asthma endotypes/phenotypes
Abstract
Background: Bronchial thermoplasty regulates structural abnormalities involved in airway narrowing in asthma. In the present study we aimed to investigate the effect of bronchial thermoplasty on histopathological bronchial structures in distinct asthma endotypes/phenotypes.
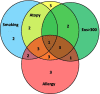
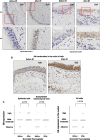
Methods: Endobronchial biopsies (n = 450) were collected from 30 patients with severe uncontrolled asthma before bronchial thermoplasty and after 3 sequential bronchial thermoplasties. Patients were classified based on blood eosinophils, atopy, allergy and smoke exposure. Tissue sections were assessed for histopathological parameters and expression of heat-shock proteins and glucocorticoid receptor. Proliferating cells were determined by Ki67-staining.
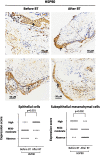
Results: In all patients, bronchial thermoplasty improved asthma control (p < 0.001), reduced airway smooth muscle (p = 0.014) and increased proliferative (Ki67 +) epithelial cells (p = 0.014). After bronchial thermoplasty, airway smooth muscle decreased predominantly in patients with T2 high asthma endotype. Epithelial cell proliferation was increased after bronchial thermoplasty in patients with low blood eosinophils (p = 0.016), patients with no allergy (p = 0.028) and patients without smoke exposure (p = 0.034). In all patients, bronchial thermoplasty increased the expression of glucocorticoid receptor in epithelial cells (p = 0.018) and subepithelial mesenchymal cells (p = 0.033) and the translocation of glucocorticoid receptor in the nucleus (p = 0.036). Furthermore, bronchial thermoplasty increased the expression of heat shock protein-70 (p = 0.002) and heat shock protein-90 (p = 0.001) in epithelial cells and decreased the expression of heat shock protein-70 (p = 0.009) and heat shock protein-90 (p = 0.002) in subepithelial mesenchymal cells. The effect of bronchial thermoplasty on the expression of heat shock proteins -70 and -90 was distinctive across different asthma endotypes/phenotypes.
Conclusions: Bronchial thermoplasty leads to a diminishment of airway smooth muscle, to epithelial cell regeneration, increased expression and activation of glucocorticoid receptor in the airways and increased expression of heat shock proteins in the epithelium. Histopathological effects appear to be distinct in different endotypes/phenotypes indicating that the beneficial effects of bronchial thermoplasty are achieved by diverse molecular targets associated with asthma endotypes/phenotypes.
Keywords: Airway smooth muscle; Asthma endotypes; Asthma phenotypes; Bronchial thermoplasty; Epithelial cell regeneration; Glucocorticoid receptor; Heat shock proteins; Severe asthma.
Conflict of interest statement
Dr. Stolz reports grants from Astra-Zeneca AG, Curetis AG, Boston Scientific, Novartis AG, GSK AG, Roche AG, Zambon, Pfizer, Schwabe Pharma AG, Vifor AG. The other authors declare that they have no competing interests related to the study.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

