Non-invasive ventilation versus high-flow nasal oxygen for postextubation respiratory failure in ICU: a post-hoc analysis of a randomized clinical trial
- PMID: 34183053
- PMCID: PMC8236736
- DOI: 10.1186/s13054-021-03621-6
Non-invasive ventilation versus high-flow nasal oxygen for postextubation respiratory failure in ICU: a post-hoc analysis of a randomized clinical trial
Abstract
Background: In intensive care units (ICUs), patients experiencing post-extubation respiratory failure have poor outcomes. The use of noninvasive ventilation (NIV) to treat post-extubation respiratory failure may increase the risk of death. This study aims at comparing mortality between patients treated with NIV alternating with high-flow nasal oxygen or high-flow nasal oxygen alone.
Methods: Post-hoc analysis of a multicenter, randomized, controlled trial focusing on patients who experienced post-extubation respiratory failure within the 7 days following extubation. Patients were classified in the NIV group or the high-flow nasal oxygen group according to oxygenation strategy used after the onset of post-extubation respiratory failure. Patients reintubated within the first hour after extubation and those promptly reintubated without prior treatment were excluded. The primary outcome was mortality at day 28 after the onset of post-extubation respiratory failure.
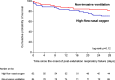
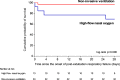
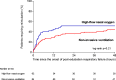
Results: Among 651 extubated patients, 158 (25%) experienced respiratory failure and 146 were included in the analysis. Mortality at day 28 was 18% (15/84) using NIV alternating with high-flow nasal oxygen and 29% (18/62) with high flow nasal oxygen alone (difference, - 11% [95% CI, - 25 to 2]; p = 0.12). Among the 46 patients with hypercapnia at the onset of respiratory failure, mortality at day 28 was 3% (1/33) with NIV and 31% (4/13) with high-flow nasal oxygen alone (difference, - 28% [95% CI, - 54 to - 6]; p = 0.006). The proportion of patients reintubated 48 h after the onset of post-extubation respiratory failure was 44% (37/84) with NIV and 52% (32/62) with high-flow nasal oxygen alone (p = 0.21).
Conclusions: In patients with post-extubation respiratory failure, NIV alternating with high-flow nasal oxygen might not increase the risk of death. Trial registration number The trial was registered at http://www.clinicaltrials.gov with the registration number NCT03121482 the 20th April 2017.
Keywords: Acute respiratory failure; Airway extubation; High-flow nasal oxygen; Noninvasive ventilation; Ventilator weaning.
Conflict of interest statement
Dr Thille reported receiving grants from the French Ministry of Health and personal fees and nonfinancial support from Fisher & Paykel Healthcare during the conduct of the study and personal fees from Maquet-Getinge, GE Healthcare, and Covidien outside the submitted work. Dr Sonneville reported receiving grants from the French Ministry of Health, the European Society of Intensive Care Medicine, and the French Society of Intensive Care Medicine and personal fees from Baxter outside the submitted work. Dr Beloncle reported receiving personal fees from Lowenstein Medical and nonfinancial support from GE Healthcare, Getinge Group, and Covidien outside the submitted work. Dr Girault reported receiving grants, personal fees, and nonfinancial support from Fisher & Paykel Healthcare during the conduct of the study and grants and nonfinancial support from ResMed outside the submitted work. Dr Ricard reported receiving travel and accommodation expenses from Fisher & Paykel Healthcare outside the submitted work. Dr Ehrmann reported receiving grants, nonfinancial support, and other funding from Fisher & Paykel Healthcare during the conduct of the study; grants, personal fees, nonfinancial support, and other funding from Aerogen; grants from Hamilton; personal fees from La Diffusion Technique Française; and personal fees from Baxter outside the submitted work. Dr Terzi reported receiving personal fees from Boehringer Ingelheim and Pfizer outside the submitted work. Dr Demoule reported receiving personal fees from Medtronic, Baxter, Hamilton, and Getinge; grants, personal fees, and nonfinancial support from Philips and Lungpacer; personal fees and nonfinancial support from Fisher & Paykel Healthcare; and grants from the French Ministry of Health and Respinor outside the submitted work. Dr Frat reported receiving personal fees and nonfinancial support from Fisher & Paykel Healthcare during the conduct of the study and personal fees and nonfinancial support from SOS Oxygen outside the submitted work. No other disclosures were reported.
Figures

References
-
- Thille AW, Muller G, Gacouin A, Coudroy R, Decavele M, Sonneville R, Beloncle F, Girault C, Dangers L, Lautrette A, et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322(15):1465–1475. doi: 10.1001/jama.2019.14901. - DOI - PMC - PubMed
-
- Subira C, Hernandez G, Vazquez A, Rodriguez-Garcia R, Gonzalez-Castro A, Garcia C, Rubio O, Ventura L, Lopez A, de la Torre MC, et al. Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: a randomized clinical trial. JAMA. 2019;321(22):2175–2182. doi: 10.1001/jama.2019.7234. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

