Evaluating the impact of a continued maternal pertussis immunisation programme in England: A modelling study and cost-effectiveness analysis
- PMID: 34183204
- PMCID: PMC8429047
- DOI: 10.1016/j.vaccine.2021.06.042
Evaluating the impact of a continued maternal pertussis immunisation programme in England: A modelling study and cost-effectiveness analysis
Abstract
Introduction: An unexpected resurgence of pertussis cases and infant deaths was observed in some countries that had switched to acellular pertussis vaccines in the primary immunisation schedule. In response to the outbreaks, maternal pertussis vaccination programmes in pregnant women have been adopted worldwide, including the USA in 2011 and the UK in 2012. Following the success of the programme in England, we evaluated the health and economic impact of stopping versus continuing the maternal pertussis immunisation to inform public health policy making.
Methods: We used a mathematical model to estimate the number of infant hospitalisations and deaths related to pertussis in England over 2019-2038. Losses in quality-adjusted life years, QALYs, were considered for infants (aged 0-2 months) who survived or died from pertussis, bereaved parents (of infants who died from pertussis), and women with pertussis (aged 20-44 years). Direct medical costs to the National Health Service included infant hospitalisations, maternal vaccinations, and disease in women. Costs and QALYs were discounted at 3.5%. Changes in the incremental cost-effectiveness ratio, ICER, were explored in sensitivity analyses.
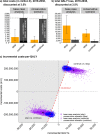
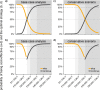
Results: The model supports continuing the maternal pertussis immunisation programme as a cost-effective intervention at an ICER of £14,500/QALY (2.5% and 97.5%-quantile: £7,300/QALY to £32,400/QALY). Stopping versus continuing the maternal programme results in an estimated mean of 972 (range 582 to 1489) versus 308 (184 to 471) infant hospitalisations annually. Results were most sensitive to the number of hospitalisations and deaths when stopping the maternal programme. At a cost-effectiveness threshold of £30,000/QALY, the probability of the maternal programme being cost-effective was 96.2%.
Conclusion: Our findings support continuing the maternal pertussis vaccination programme as otherwise higher levels of disease activity and infant mortality are expected to return. These results have led policy makers to decide to continue the maternal programme in the UK routine immunisation schedule.
Keywords: Economic evaluation; Maternal vaccination; Mathematical model; Pertussis; Public health; Resurgence.
Copyright © 2021. Published by Elsevier Ltd.
Figures


References
-
- Campbell H., Gupta S., Dolan G.P. Review of vaccination in pregnancy to prevent pertussis in early infancy. J Med Microbiol. 2018;67(10):1426–1456. - PubMed
-
- World Health Organization (WHO). Revised guidance on the choice of pertussis vaccines: July 2014. Releve epidemiologique hebdomadaire 2014; 89(30): 337-40. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

