The first report on coronavirus disease 2019 (COVID-19) vaccine refusal by patients with solid cancer in Italy: Early data from a single-institute survey
- PMID: 34183225
- PMCID: PMC8149194
- DOI: 10.1016/j.ejca.2021.05.006
The first report on coronavirus disease 2019 (COVID-19) vaccine refusal by patients with solid cancer in Italy: Early data from a single-institute survey
Abstract
Introduction: Patients with cancer have an increased risk of complications from coronavirus disease 2019 (COVID-19) infection, including death, and thus, they were considered as high-priority subjects for COVID-19 vaccination. We report on the compliance with the COVID-19 vaccine of patients affected by solid tumours.
Materials and methods: Patients with cancer afferent to Medical Oncology 1 Unit of Regina Elena National Cancer Institute in Rome were considered eligible for vaccination if they were receiving systemic immunosuppressive antitumor treatment or received it in the last 6 months or having an uncontrolled advanced disease. The Pfizer BNT162b2 vaccine was proposed to all candidates via phone or during a scheduled visit. The reasons for refusal were collected by administrating a 6-item multiple-choice questionnaire.
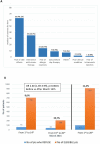
Results: From 1st March to 20th March 2021, of 914 eligible patients, 102 refused vaccination (11.2%, 95% confidence interval [CI] 9.1-13.2). The most frequent (>10%) reasons reported were concerns about vaccine-related adverse events (48.1%), negative interaction with concomitant antitumor therapy (26.7%), and the fear of allergic reaction (10.7%). The refusal rate (RR) after 15th March (date of AstraZeneca-AZD1222 suspension) was more than doubled compared with the RR observed before (19.7% versus 8.6%, odds ratio [OR] 2.60, 95% CI 1.69-3.99; P < 0.0001). ECOG-PS 2 was associated with higher RR compared with ECOG-PS 0-1 (OR 2.94, 95% CI 1.04-8.34; P = 0.04). No statistically significant differences in RR according to other clinical characteristics were found.
Conclusions: Our experience represents the first worldwide report on the adherence of patients with cancer to COVID-19 vaccination and underlines how regulatory decisions and media news spreading could influence the success of the campaign.
Keywords: BNT162b2; COVID-19; Cancer patients; Public health; Refusal; SARS-CoV-2; Vaccine.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement V.D.N. received speaker’s fee by AstraZeneca, MSD, BMS, Istituto Gentili, Boehringer Ingelheim. V.D.N. received grant consultancies by AstraZeneca, MSD, BMS, Boehringer Ingelheim and travel fee from MSD and Boehringer Ingelheim. V.D.N. received institutional research grants from Roche. F.C. was a member of advisory board of GSK, Roche, AstraZeneca, and Eli-Lilly. F.C. received speaker’s fee by GSK, Roche, AstraZeneca, Eli-Lilly, Novartis, Amgen, Pfizer, MSD, BMS, Astellas, and Eli-Lilly. All remaining authors have declared no conflicts of interest.
Figures
Comment in
-
Adherence to COVID-19 vaccines in cancer patients: promote it and make it happen!Eur J Cancer. 2021 Aug;153:257-259. doi: 10.1016/j.ejca.2021.05.007. Epub 2021 May 24. Eur J Cancer. 2021. PMID: 34147290 Free PMC article. No abstract available.
-
Refusal of anti-coronavirus disease 2019 vaccination in cancer patients: Is there a difference between the sexes?Eur J Cancer. 2021 Sep;155:54-55. doi: 10.1016/j.ejca.2021.06.048. Epub 2021 Jul 21. Eur J Cancer. 2021. PMID: 34352570 Free PMC article. No abstract available.
-
Re: The first report on coronavirus disease 2019 vaccine refusal by patients with cancer in Italy: Early data from a single-institute survey: Consideration of recent findings on COVID-19 vaccine adherence in cancer patients.Eur J Cancer. 2021 Nov;158:251-252. doi: 10.1016/j.ejca.2021.07.048. Epub 2021 Sep 25. Eur J Cancer. 2021. PMID: 34649763 Free PMC article. No abstract available.
-
Reply to the letter entitled: Re: The first report on Covid-19 vaccine refusal by patients with cancer in Italy: Early data from a single-institute survey.Eur J Cancer. 2021 Nov;158:253-254. doi: 10.1016/j.ejca.2021.09.007. Epub 2021 Sep 25. Eur J Cancer. 2021. PMID: 34663560 Free PMC article. No abstract available.
References
-
- Agenzia Italiana del Farmaco. COVID-19 vaccines communications. https://www.aifa.gov.it/en/web/guest/-/covid-19-vaccine-astrazeneca-bene.... Accessed April 15th, 2021.
-
- Open Online. Quanti sono i medici e infermieri No Vax negli ospedali? 35 mila non risultano ancora vaccinati: cosa non torna nelle stime basse dei sindacati https://www.open.online/2021/03/27/covid-19-medici-infermieri-no-vax-cos.... Accessed April 15th, 2021.
-
- Corriere della Sera. Vaccini, il sondaggio di Pagnoncelli: italiani ora più cauti, ma il 52% è pronto a farlo subito https://www.corriere.it/politica/21_marzo_20/vaccini-italiani-ora-piu-ca.... Accessed April 15th, 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

