Safety and Efficacy of the Amplatzer Septal Occluder: A Systematic Review and Meta-Analysis
- PMID: 34183276
- PMCID: PMC10909392
- DOI: 10.1016/j.carrev.2021.06.002
Safety and Efficacy of the Amplatzer Septal Occluder: A Systematic Review and Meta-Analysis
Abstract
Objectives: To assess the safety and efficacy of the Amplatzer Septal Occluder in the closure of secundum type atrial septal defects.
Background: The Amplatzer Septal Occluder (ASO; Abbott, St. Paul, MN) is an FDA-approved device for percutaneous closure of secundum type atrial septal defects (ASD). Previous small cohort trials have shown a favorable safety and technical efficacy profile.
Methods: We conducted a systemic review and meta-analysis of all prospective case series and controlled trials that evaluated the ASO's safety and implant efficacy. The primary endpoint was the technical success rate of implantations. Secondary outcomes included proportions of arrhythmias and embolism specific-adverse events.
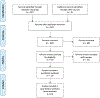
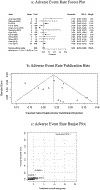
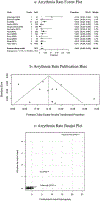
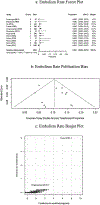
Results: We included a total of 12 studies with 2972 patients. The ratio of device implantation was 2:1 by sex [female: male]. Pooled technical success rate of implantation was 98% (95% CI: 0.968-0.990, P < 0.01). The cumulative adverse event rate was 5.1% (95% CI: 0.035-0.068, P < 0.01), which included arrhythmia and embolism specific adverse event rates of 1.8% (95% CI: 0.007-0.032, P < 0.01) and 0.7% (95% CI: 0.002-0.013, P < 0.01), respectively. Sensitivity analysis did not significantly affect pooled outcomes for success rate and adverse events; both forest plot and Begg's and Egger's regression tests supported symmetricity.
Conclusion: A high likelihood of technical success can be expected when implanting the ASO in secundum type ASDs. Adverse event rates are expected for one in twenty patients, and thus, our results support the safe use of ASO in secundum type ASDs closure.
Condensed abstract: The AMPLATZER Septal Occluder is an FDA-approved device for percutaneous closure of secundum type atrial septal defects (ASD). We conducted a systemic review and meta-analysis of all prospective case series and controlled trials that evaluated the ASO's safety and implant efficacy. We included a total of 12 studies with 2972 patients. Pooled technical success rate of implantation was 98% (P < 0.01). The cumulative adverse event rate was 5.1% (P < 0.01), 1.8% (P < 0.01) rate of arrhythmias, and 0.7% (P < 0.01) rate of embolisms. A high likelihood of technical success can be expected with a low rate of adverse events.
Keywords: Amplatzer; Congenital heart defect; Secundum type atrial septal defect; Septal occluder.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors report no conflicts of interest regarding the content herein.
Figures

Similar articles
-
Transcatheter Closure of Atrial Septal Defect: A Review of Currently Used Devices.Cureus. 2023 Jun 8;15(6):e40132. doi: 10.7759/cureus.40132. eCollection 2023 Jun. Cureus. 2023. PMID: 37425612 Free PMC article. Review.
-
Long-Term Results of the Atrial Septal Defect Occluder ASSURED Trial for Combined Pivotal/Continued Access Cohorts.JACC Cardiovasc Interv. 2024 Oct 14;17(19):2274-2283. doi: 10.1016/j.jcin.2024.07.013. Epub 2024 Sep 18. JACC Cardiovasc Interv. 2024. PMID: 39297855 Clinical Trial.
-
Clinical results of large secundum atrial septal defect closure in adult using percutaneous transcatheter Cocoon atrial septal occluder.J Med Assoc Thai. 2013 Sep;96(9):1127-34. J Med Assoc Thai. 2013. PMID: 24163987
-
Use of the GORE® CARDIOFORM Septal Occluder for percutaneous closure of secundum atrial septal defects: Results of the multicenter U.S. IDE trial.Catheter Cardiovasc Interv. 2020 Jun 1;95(7):1296-1304. doi: 10.1002/ccd.28814. Epub 2020 Feb 28. Catheter Cardiovasc Interv. 2020. PMID: 32108423
-
Systematic review of multiple versus single device closure of Secundum atrial septal defects in adults.Cardiovasc Revasc Med. 2024 Jan;58:90-97. doi: 10.1016/j.carrev.2023.07.027. Epub 2023 Aug 3. Cardiovasc Revasc Med. 2024. PMID: 37596193
Cited by
-
A multi-center trial on efficacy and safety of the LifeTech CeraFlexTM ASD occluder for transcatheter closure in patients with secundum atrial septal defects.Cardiovasc Diagn Ther. 2022 Aug;12(4):475-484. doi: 10.21037/cdt-21-798. Cardiovasc Diagn Ther. 2022. PMID: 36033225 Free PMC article.
-
The Prevalence of and Predisposing Factors for Late Atrial Arrhythmias after Transcatheter Closure of Secundum Atrial Septal Defects in Children.J Clin Med. 2023 May 28;12(11):3717. doi: 10.3390/jcm12113717. J Clin Med. 2023. PMID: 37297912 Free PMC article.
-
Safety and one-year follow-up analysis of percutaneous ASD closure at a tertiary care hospital.Indian Heart J. 2025 May-Jun;77(3):199-203. doi: 10.1016/j.ihj.2025.03.011. Epub 2025 Mar 27. Indian Heart J. 2025. PMID: 40157568 Free PMC article.
-
An Amplatzer Septal Occluder Trapped in the Left Ventricular Outflow Tract: A Case Report.Cureus. 2024 Nov 7;16(11):e73244. doi: 10.7759/cureus.73244. eCollection 2024 Nov. Cureus. 2024. PMID: 39650882 Free PMC article.
References
-
- Mills NL, King TD. Nonoperative closure of left-to-right shunts. J Thorac Cardiovasc Surg. 1976;72(3):371–8. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

