COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants
- PMID: 34183681
- PMCID: PMC8239026
- DOI: 10.1038/s41467-021-24285-4
COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants
Abstract
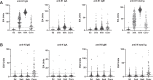
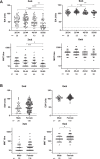
As SARS-CoV-2 has been circulating for over a year, dozens of vaccine candidates are under development or in clinical use. The BNT162b2 mRNA COVID-19 vaccine induces spike protein-specific neutralizing antibodies associated with protective immunity. The emergence of the B.1.1.7 and B.1.351 variants has raised concerns of reduced vaccine efficacy and increased re-infection rates. Here we show, that after the second dose, the sera of BNT162b2-vaccinated health care workers (n = 180) effectively neutralize the SARS-CoV-2 variant with the D614G substitution and the B.1.1.7 variant, whereas the neutralization of the B.1.351 variant is five-fold reduced. Despite the reduction, 92% of the seronegative vaccinees have a neutralization titre of >20 for the B.1.351 variant indicating some protection. The vaccinees' neutralization titres exceeded those of recovered non-hospitalized COVID-19 patients. Our work provides evidence that the second dose of the BNT162b2 vaccine induces cross-neutralization of at least some of the circulating SARS-CoV-2 variants.
Conflict of interest statement
The authors declare no competing interests.
Figures




Comment in
-
Myocarditis associated with COVID‑19 vaccination in three male teenagers.Pol Arch Intern Med. 2022 Feb 28;132(2):16160. doi: 10.20452/pamw.16160. Epub 2021 Dec 1. Pol Arch Intern Med. 2022. PMID: 34851078 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

