Efficient long-range conduction in cable bacteria through nickel protein wires
- PMID: 34183682
- PMCID: PMC8238962
- DOI: 10.1038/s41467-021-24312-4
Efficient long-range conduction in cable bacteria through nickel protein wires
Abstract
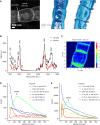
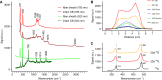
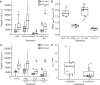
Filamentous cable bacteria display long-range electron transport, generating electrical currents over centimeter distances through a highly ordered network of fibers embedded in their cell envelope. The conductivity of these periplasmic wires is exceptionally high for a biological material, but their chemical structure and underlying electron transport mechanism remain unresolved. Here, we combine high-resolution microscopy, spectroscopy, and chemical imaging on individual cable bacterium filaments to demonstrate that the periplasmic wires consist of a conductive protein core surrounded by an insulating protein shell layer. The core proteins contain a sulfur-ligated nickel cofactor, and conductivity decreases when nickel is oxidized or selectively removed. The involvement of nickel as the active metal in biological conduction is remarkable, and suggests a hitherto unknown form of electron transport that enables efficient conduction in centimeter-long protein structures.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Meysman, F. J. R. Cable bacteria take a new breath using long-distance electricity. Trends Microbiol.10.1016/j.tim.2017.10.011 (2018). - PubMed

