A rapid near-patient detection system for SARS-CoV-2 using saliva
- PMID: 34183720
- PMCID: PMC8238998
- DOI: 10.1038/s41598-021-92677-z
A rapid near-patient detection system for SARS-CoV-2 using saliva
Abstract
The highly infectious nature of SARS-CoV-2 necessitates the use of widespread testing to control the spread of the virus. Presently, the standard molecular testing method (reverse transcriptase-polymerase chain reaction, RT-PCR) is restricted to the laboratory, time-consuming, and costly. This increases the turnaround time for getting test results. This study sought to develop a rapid, near-patient saliva-based test for COVID-19 (Saliva-Dry LAMP) with similar accuracy to that of standard RT-PCR tests. A lyophilized dual-target reverse transcription-loop-mediated isothermal amplification (RT-LAMP) test with fluorometric detection by the naked eye was developed. The assay relies on dry reagents that are room temperature stable. A device containing a centrifuge, heat block, and blue LED light system was manufactured to reduce the cost of performing the assay. This test has a limit of detection of 1 copy/µL and achieved a positive percent agreement of 100% [95% CI 88.43% to 100.0%] and a negative percent agreement of 96.7% [95% CI 82.78-99.92%] relative to a reference standard test. Saliva-Dry LAMP can be completed in 105 min. Precision, cross-reactivity, and interfering substances analysis met international regulatory standards. The combination of ease of sample collection, dry reagents, visual detection, low capital equipment cost, and excellent analytical sensitivity make Saliva-Dry LAMP particularly useful for resource-limited settings.
Conflict of interest statement
DRP is scientific advisor to Illucidx Inc., a University of Calgary start-up company supported by Innovate Calgary, which holds patents related to LAMP technology. ANM is a technical advisor to Illucidx Inc. All other authors declare no conflicts of interest.
Figures
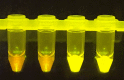

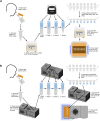
References
-
- Sarata AK. COVID-19 testing: Key issues. Congr. Res. Serv. 2020;2:1–3.
-
- Centres for Disease Control and Prevention. Real-time RT-PCR Primers and Probes for COVID-19 | CDC. cdc.gov (2020). Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.... (Accessed: 3rd November 2020)
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

