Glutamine supplementation stimulates cell proliferation in skeletal muscle and cultivated myogenic cells of low birth weight piglets
- PMID: 34183762
- PMCID: PMC8239033
- DOI: 10.1038/s41598-021-92959-6
Glutamine supplementation stimulates cell proliferation in skeletal muscle and cultivated myogenic cells of low birth weight piglets
Abstract
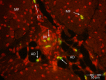
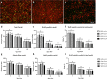
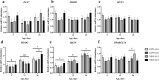
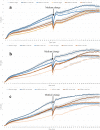
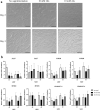
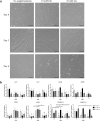
Muscle growth of low birth weight (LBW) piglets may be improved with adapted nutrition. This study elucidated effects of glutamine (Gln) supplementation on the cellular muscle development of LBW and normal birth weight (NBW) piglets. Male piglets (n = 144) were either supplemented with 1 g Gln/kg body weight or an isonitrogeneous amount of alanine (Ala) between postnatal day 1 and 12 (dpn). Twelve piglets per group were slaughtered at 5, 12 and 26 dpn, one hour after injection with Bromodeoxyuridine (BrdU, 12 mg/kg). Muscle samples were collected and myogenic cells were isolated and cultivated. Expression of muscle growth related genes was quantified with qPCR. Proliferating, BrdU-positive cells in muscle sections were detected with immunohistochemistry indicating different cell types and decreasing proliferation with age. More proliferation was observed in muscle tissue of LBW-GLN than LBW-ALA piglets at 5 dpn, but there was no clear effect of supplementation on related gene expression. Cell culture experiments indicated that Gln could promote cell proliferation in a dose dependent manner, but expression of myogenesis regulatory genes was not altered. Overall, Gln supplementation stimulated cell proliferation in muscle tissue and in vitro in myogenic cell culture, whereas muscle growth regulatory genes were barely altered.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Acute and persistent effects of oral glutamine supplementation on growth, cellular proliferation, and tight junction protein transcript abundance in jejunal tissue of low and normal birthweight pre-weaning piglets.PLoS One. 2024 Jan 2;19(1):e0296427. doi: 10.1371/journal.pone.0296427. eCollection 2024. PLoS One. 2024. PMID: 38165864 Free PMC article.
-
Identification and Quantification of Proliferating Cells in Skeletal Muscle of Glutamine Supplemented Low- and Normal-Birth-Weight Piglets.Cells. 2023 Feb 11;12(4):580. doi: 10.3390/cells12040580. Cells. 2023. PMID: 36831247 Free PMC article.
-
Glutamine supplementation moderately affects growth, plasma metabolite and free amino acid patterns in neonatal low birth weight piglets.Br J Nutr. 2022 Dec 28;128(12):2330-2340. doi: 10.1017/S0007114522000459. Epub 2022 Feb 11. Br J Nutr. 2022. PMID: 35144703 Free PMC article.
-
The effect of glutamine supplementation in patients following elective surgery and accidental injury.J Nutr. 2001 Sep;131(9 Suppl):2543S-9S; discussion 2550S-1S. doi: 10.1093/jn/131.9.2543S. J Nutr. 2001. PMID: 11533310 Review.
-
Creatine Supplementation and Skeletal Muscle Metabolism for Building Muscle Mass- Review of the Potential Mechanisms of Action.Curr Protein Pept Sci. 2017;18(12):1273-1287. doi: 10.2174/1389203718666170606105108. Curr Protein Pept Sci. 2017. PMID: 28595527 Review.
Cited by
-
Acute and persistent effects of oral glutamine supplementation on growth, cellular proliferation, and tight junction protein transcript abundance in jejunal tissue of low and normal birthweight pre-weaning piglets.PLoS One. 2024 Jan 2;19(1):e0296427. doi: 10.1371/journal.pone.0296427. eCollection 2024. PLoS One. 2024. PMID: 38165864 Free PMC article.
-
Cell culture-derived extracellular vesicles: Considerations for reporting cell culturing parameters.J Extracell Biol. 2023 Oct 16;2(10):e115. doi: 10.1002/jex2.115. eCollection 2023 Oct. J Extracell Biol. 2023. PMID: 38939735 Free PMC article.
-
Identification and Quantification of Proliferating Cells in Skeletal Muscle of Glutamine Supplemented Low- and Normal-Birth-Weight Piglets.Cells. 2023 Feb 11;12(4):580. doi: 10.3390/cells12040580. Cells. 2023. PMID: 36831247 Free PMC article.
-
Selected Nutrients to Oppose Muscle Disuse Following Arthroscopic Orthopedic Surgery: A Narrative Review.Nutrients. 2025 Apr 5;17(7):1273. doi: 10.3390/nu17071273. Nutrients. 2025. PMID: 40219030 Free PMC article. Review.
-
Skeletal Muscle Metabolism Is Dynamic during Porcine Postnatal Growth.Metabolites. 2024 Jun 26;14(7):357. doi: 10.3390/metabo14070357. Metabolites. 2024. PMID: 39057680 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

