A species-specific functional module controls formation of pollen apertures
- PMID: 34183783
- PMCID: PMC8292223
- DOI: 10.1038/s41477-021-00951-9
A species-specific functional module controls formation of pollen apertures
Abstract
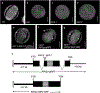
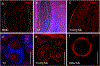
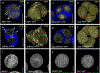
Pollen apertures are an interesting model for the formation of specialized plasma-membrane domains. The plant-specific protein INP1 serves as a key aperture factor in such distantly related species as Arabidopsis, rice and maize. Although INP1 orthologues probably play similar roles throughout flowering plants, they show substantial sequence divergence and often cannot substitute for each other, suggesting that INP1 might require species-specific partners. Here, we present a new aperture factor, INP2, which satisfies the criteria for being a species-specific partner for INP1. Both INP proteins display similar structural features, including the plant-specific DOG1 domain, similar patterns of expression and mutant phenotypes, as well as signs of co-evolution. These proteins interact with each other in a species-specific manner and can restore apertures in a heterologous system when both are expressed but not when expressed individually. Our findings suggest that the INP proteins form a species-specific functional module that underlies formation of pollen apertures.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Figures








References
-
- Furness CA & Rudall PJ Pollen aperture evolution – a crucial factor for eudicot success? Trends Plant Sci. 9, 154–158 (2004). - PubMed
-
- Zhou Y & Dobritsa AA Formation of aperture sites on the pollen surface as a model for development of distinct cellular domains. Plant Science 288, 110222 (2019). - PubMed
-
- Heslop-Harrison J An interpretation of the hydrodynamics of pollen. Am. J. Bot 66, 737–743 (1979).
-
- Vieira AM & Feijó JA Hydrogel control of water uptake by pectins during in vitro pollen hydration of Eucalyptus globulus. Am. J. Bot 103, 437–451 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

