Single-molecule imaging of LexA degradation in Escherichia coli elucidates regulatory mechanisms and heterogeneity of the SOS response
- PMID: 34183814
- PMCID: PMC7611437
- DOI: 10.1038/s41564-021-00930-y
Single-molecule imaging of LexA degradation in Escherichia coli elucidates regulatory mechanisms and heterogeneity of the SOS response
Abstract
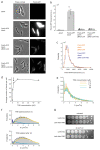
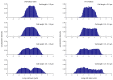
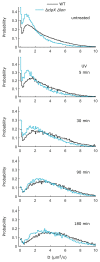
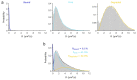
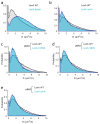
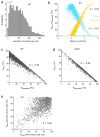
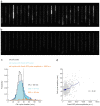
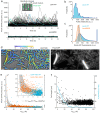
The bacterial SOS response represents a paradigm of gene networks controlled by a master transcriptional regulator. Self-cleavage of the SOS repressor LexA induces a wide range of cell functions that are critical for survival and adaptation when bacteria experience stress conditions1 including DNA repair2, mutagenesis3,4, horizontal gene transfer5-7, filamentous growth and the induction of bacterial toxins8-12, toxin-antitoxin systems13, virulence factors6,14 and prophages15-17. SOS induction is also implicated in biofilm formation and antibiotic persistence11,18-20. Considering the fitness burden of these functions, it is surprising that the expression of LexA-regulated genes is highly variable across cells10,21-23 and that cell subpopulations induce the SOS response spontaneously even in the absence of stress exposure9,11,12,16,24,25. Whether this reflects a population survival strategy or a regulatory inaccuracy is unclear, as are the mechanisms underlying SOS heterogeneity. Here, we developed a single-molecule imaging approach based on a HaloTag fusion to directly monitor LexA in live Escherichia coli cells, demonstrating the existence of three main states of LexA: DNA-bound stationary molecules, free LexA and degraded LexA species. These analyses elucidate the mechanisms by which DNA binding and degradation of LexA regulate the SOS response in vivo. We show that self-cleavage of LexA occurs frequently throughout the population during unperturbed growth, rather than being restricted to a subpopulation of cells. This causes substantial cell-to-cell variation in LexA abundances. LexA variability underlies SOS gene-expression heterogeneity and triggers spontaneous SOS pulses, which enhance bacterial survival in anticipation of stress.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Baharoglu Z, Mazel D. SOS, the formidable strategy of bacteria against aggressions. FEMS Microbiol Rev. 2014;38:1126–1145. - PubMed
-
- Schlacher K, Goodman MF. Lessons from 50 years of SOS DNA-damage-induced mutagenesis. Nat Rev Mol Cell Biol. 2007;8:587–594. - PubMed
-
- Beaber JW, Hochhut B, Waldor MK. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature. 2004;427:72–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

