Fully implantable and bioresorbable cardiac pacemakers without leads or batteries
- PMID: 34183859
- PMCID: PMC9270064
- DOI: 10.1038/s41587-021-00948-x
Fully implantable and bioresorbable cardiac pacemakers without leads or batteries
Abstract
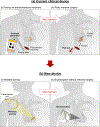
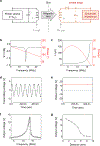
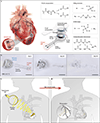
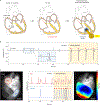
Temporary cardiac pacemakers used in periods of need during surgical recovery involve percutaneous leads and externalized hardware that carry risks of infection, constrain patient mobility and may damage the heart during lead removal. Here we report a leadless, battery-free, fully implantable cardiac pacemaker for postoperative control of cardiac rate and rhythm that undergoes complete dissolution and clearance by natural biological processes after a defined operating timeframe. We show that these devices provide effective pacing of hearts of various sizes in mouse, rat, rabbit, canine and human cardiac models, with tailored geometries and operation timescales, powered by wireless energy transfer. This approach overcomes key disadvantages of traditional temporary pacing devices and may serve as the basis for the next generation of postoperative temporary pacing technology.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures







Comment in
-
Fully bioresorbable, battery-free pacemakers.Nat Rev Cardiol. 2021 Sep;18(9):611. doi: 10.1038/s41569-021-00600-w. Nat Rev Cardiol. 2021. PMID: 34244679 No abstract available.
References
-
- Waldo AL, Wells JLJ, Cooper TB & MacLean WA Temporary cardiac pacing: applications and techniques in the treatment of cardiac arrhythmias. Prog. Cardiovasc. Dis. 23, 451–474 (1981). - PubMed
-
- Zoll PM et al. External noninvasive temporary cardiac pacing: clinical trials. Circulation 71, 937–944 (1985). - PubMed
-
- Curtis JJ et al. A critical look at temporary ventricular pacing following cardiac surgery. Surgery 82, 888–893 (1977). - PubMed
-
- Wilhelm MJ et al. Cardiac pacemaker infection: surgical management with and without extracorporeal circulation. Ann. Thorac. Surg. 64, 1707–1712 (1997). - PubMed
-
- Choo MH et al. Permanent pacemaker infections: characterization and management. Am. J. Cardiol. 48, 559–564 (1981). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

