COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects
- PMID: 34184164
- PMCID: PMC8238037
- DOI: 10.1007/s10456-021-09805-6
COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects
Abstract
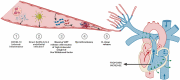
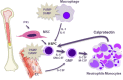
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is presenting as a systemic disease associated with vascular inflammation and endothelial injury. Severe forms of SARS-CoV-2 infection induce acute respiratory distress syndrome (ARDS) and there is still an ongoing debate on whether COVID-19 ARDS and its perfusion defect differs from ARDS induced by other causes. Beside pro-inflammatory cytokines (such as interleukin-1 β [IL-1β] or IL-6), several main pathological phenomena have been seen because of endothelial cell (EC) dysfunction: hypercoagulation reflected by fibrin degradation products called D-dimers, micro- and macrothrombosis and pathological angiogenesis. Direct endothelial infection by SARS-CoV-2 is not likely to occur and ACE-2 expression by EC is a matter of debate. Indeed, endothelial damage reported in severely ill patients with COVID-19 could be more likely secondary to infection of neighboring cells and/or a consequence of inflammation. Endotheliopathy could give rise to hypercoagulation by alteration in the levels of different factors such as von Willebrand factor. Other than thrombotic events, pathological angiogenesis is among the recent findings. Overexpression of different proangiogenic factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (FGF-2) or placental growth factors (PlGF) have been found in plasma or lung biopsies of COVID-19 patients. Finally, SARS-CoV-2 infection induces an emergency myelopoiesis associated to deregulated immunity and mobilization of endothelial progenitor cells, leading to features of acquired hematological malignancies or cardiovascular disease, which are discussed in this review. Altogether, this review will try to elucidate the pathophysiology of thrombotic complications, pathological angiogenesis and EC dysfunction, allowing better insight in new targets and antithrombotic protocols to better address vascular system dysfunction. Since treating SARS-CoV-2 infection and its potential long-term effects involves targeting the vascular compartment and/or mobilization of immature immune cells, we propose to define COVID-19 and its complications as a systemic vascular acquired hemopathy.
© 2021. The Author(s), under exclusive licence to Springer Nature B.V.
Conflict of interest statement
CK reports personal fees from Maquet, Xenios, and Bayer, as well as non-financial support from Speaker of the German register of ICUs and grants from the German Ministry of Research and Education.
Figures


References
-
- Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

