Molecular cell identities in the mediodorsal thalamus of infant mice and marmoset
- PMID: 34184265
- PMCID: PMC8714865
- DOI: 10.1002/cne.25203
Molecular cell identities in the mediodorsal thalamus of infant mice and marmoset
Abstract
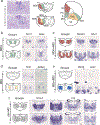
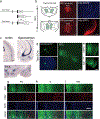
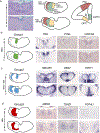
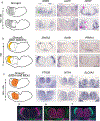
The mediodorsal thalamus (MD) is a higher-order nucleus located within the central thalamus in many mammalian species. Emerging evidence from MD lesions and tracer injections suggests that the MD is reciprocally connected to the prefrontal cortex (PFC) and plays an essential role in specific cognitive processes and tasks. MD subdivisions (medial, central, and lateral) are poorly segregated at the molecular level in rodents, leading to a lack of MD subdivision-specific Cre driver mice. Moreover, this lack of molecular identifiers hinders MD subdivision- and cell-type-specific circuit formation and function analysis. Therefore, using publicly available databases, we explored molecules separately expressed in MD subdivisions. In addition to MD subdivision markers, we identified several genes expressed in a subdivision-specific combination and classified them. Furthermore, after developing medial MD (MDm) or central MD (MDc) region-specific Cre mouse lines, we identified diverse region- and layer-specific PFC projection patterns. Comparison between classified MD marker genes in mice and common marmosets, a nonhuman primate model, revealed diverging gene expression patterns. These results highlight the species-specific organization of cell types and their projections in the MD thalamus.
Keywords: cognition; evolution; mediodorsal thalamus; nonhuman primate; prefrontal cortex.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
Figures

Similar articles
-
High-Resolution Tractography Protocol to Investigate the Pathways between Human Mediodorsal Thalamic Nucleus and Prefrontal Cortex.J Neurosci. 2023 Nov 15;43(46):7780-7798. doi: 10.1523/JNEUROSCI.0721-23.2023. Epub 2023 Sep 14. J Neurosci. 2023. PMID: 37709539 Free PMC article.
-
The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys.J Comp Neurol. 1993 Nov 1;337(1):1-31. doi: 10.1002/cne.903370102. J Comp Neurol. 1993. PMID: 7506270
-
The mediodorsal thalamus as a higher order thalamic relay nucleus important for learning and decision-making.Neurosci Biobehav Rev. 2015 Jul;54:76-88. doi: 10.1016/j.neubiorev.2015.03.001. Epub 2015 Mar 7. Neurosci Biobehav Rev. 2015. PMID: 25757689 Review.
-
Differential Roles of Mediodorsal Nucleus of the Thalamus and Prefrontal Cortex in Decision-Making and State Representation in a Cognitive Control Task Measuring Deficits in Schizophrenia.J Neurosci. 2020 Feb 19;40(8):1650-1667. doi: 10.1523/JNEUROSCI.1703-19.2020. Epub 2020 Jan 15. J Neurosci. 2020. PMID: 31941665 Free PMC article.
-
Finding prefrontal cortex in the rat.Brain Res. 2016 Aug 15;1645:1-3. doi: 10.1016/j.brainres.2016.02.002. Epub 2016 Feb 8. Brain Res. 2016. PMID: 26867704 Review.
Cited by
-
Molecular architecture of primate specific neural circuit formation.Res Sq [Preprint]. 2024 Mar 22:rs.3.rs-4082064. doi: 10.21203/rs.3.rs-4082064/v1. Res Sq. 2024. PMID: 38562839 Free PMC article. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

