In vitro and in vivo safety studies indicate that R15, a synthetic polyarginine peptide, could safely reverse the effects of unfractionated heparin
- PMID: 34184429
- PMCID: PMC8409304
- DOI: 10.1002/2211-5463.13240
In vitro and in vivo safety studies indicate that R15, a synthetic polyarginine peptide, could safely reverse the effects of unfractionated heparin
Abstract
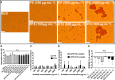
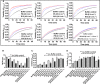
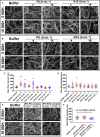
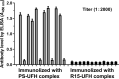
Unfractionated heparin (UFH) is an anionic glycosaminoglycan that is widely used to prevent blood clotting. However, in certain cases, unwanted side effects can require it to be neutralized. Protamine sulfate (PS), a basic peptide rich in arginine, is the only approved antagonist for UFH neutralization. Many adverse reactions occur with the clinical application of PS, including systemic hypotension, pulmonary hypertension, and anaphylaxis. We previously described R15, a linear peptide composed of 15 arginine molecules, as a potential UFH antagonist. In this study, the in-depth safety of R15 was explored to reveal its merits and associated risks in comparison with PS. In vitro safety studies investigated the interactions of R15 with erythrocytes, fibrin, complement, and rat plasma. In vivo safety studies explored potential toxicity and immunogenicity of R15 and the UFH-R15 complex. Results showed that both PS and R15 can induce erythrocyte aggregation, thicken fibrin fibers, activate complement, and cause anticoagulation in a concentration-dependent manner. However, those influences weakened in whole blood or in live animals and were avoided when R15 was in a complex with UFH. We found dramatically enhanced complement activation when there was excess UFH in analyses involving UFH-PS complexes, and a slight increase in those involving UFH-R15 complexes. Within 2 h, R15 was degraded in rat plasma in vitro, whereas PS was not. Enhanced creatinine was found after a single intravenous injection of PS or R15 (900 U·kg-1 , body weight), suggesting possible abnormal renal function. The UFH-PS complex, but not the UFH-R15 complex, exhibited obvious immunogenicity. In conclusion, R15 is nonimmunogenic and potentially safe at a therapeutic dose to reverse the effects of UFH.
Keywords: heparin reversal; heparin substitutes; immunogenicity; polyarginine; protamine.
© 2021 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
New synthetic peptide with efficacy for heparin reversal and low toxicity and immunogenicity in comparison to protamine sulfate.Biochem Biophys Res Commun. 2015 Nov 20;467(3):497-502. doi: 10.1016/j.bbrc.2015.10.020. Epub 2015 Oct 9. Biochem Biophys Res Commun. 2015. PMID: 26456655
-
Neutralization of DHG, a new depolymerized holothurian glycosaminoglycan, by protamine sulfate and platelet factor 4.Haemostasis. 1997 Jul-Aug;27(4):174-83. doi: 10.1159/000217453. Haemostasis. 1997. PMID: 9483172
-
Differential Neutralization of Unfractionated Heparin and Enoxaparin by Andexanet Alfa.Clin Appl Thromb Hemost. 2022 Jan-Dec;28:10760296221099934. doi: 10.1177/10760296221099934. Clin Appl Thromb Hemost. 2022. PMID: 35535398 Free PMC article.
-
Andexanet Alfa, the Possible Alternative to Protamine for Reversal of Unfractionated Heparin.Ann Pharmacother. 2021 Feb;55(2):261-264. doi: 10.1177/1060028020943160. Epub 2020 Jul 15. Ann Pharmacother. 2021. PMID: 32667214 Review.
-
Protamine and Heparin Interactions: A Narrative Review.Ann Card Anaesth. 2024 Jul 1;27(3):202-212. doi: 10.4103/aca.aca_117_23. Epub 2024 Jul 4. Ann Card Anaesth. 2024. PMID: 38963354 Free PMC article. Review.
References
-
- Ando T, Yamasaki M and Suzuki K (1973) Protamines. Isolation, characterization, structure and function. Mol Biol Biochem Biophys 12, 1–114. - PubMed
-
- Shapira N, Schaff HV, Piehler JM, White RD, Sill JC and Pluth JR (1982) Cardiovascular effects of protamine sulfate in man. J Thorac Cardiovasc Surg 84, 505–514. - PubMed
-
- Horrow JC (1985) Protamine: a review of its toxicity. Anesth Analg 64, 348–361. - PubMed
-
- Leung LWM, Gallagher MM, Evranos B, Bolten J, Madden BP, Wright S and Kaba RA (2019) Cardiac arrest following protamine administration: a case series. Europace 21, 886–892. - PubMed
-
- Zöllner H, Jouni R, Panzer S, Khadour A, Janzen L, Wesche J, ten Berg M, Schellong S, Heinken A, Greinacher Aet al. (2017) Platelet activation in the presence of neutral protamine Hagedorn insulin: a new feature of antibodies against protamine/heparin complexes. J Thromb Haemost 15, 176–184. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

