Ivermectin treatment in humans for reducing malaria transmission
- PMID: 34184757
- PMCID: PMC8240090
- DOI: 10.1002/14651858.CD013117.pub2
Ivermectin treatment in humans for reducing malaria transmission
Abstract
Background: Malaria is transmitted through the bite of Plasmodium-infected adult female Anopheles mosquitoes. Ivermectin, an anti-parasitic drug, acts by killing mosquitoes that are exposed to the drug while feeding on the blood of people (known as blood feeds) who have ingested the drug. This effect on mosquitoes has been demonstrated by individual randomized trials. This effect has generated interest in using ivermectin as a tool for malaria control.
Objectives: To assess the effect of community administration of ivermectin on malaria transmission.
Search methods: We searched the Cochrane Infectious Diseases Group (CIDG) Specialized Register, CENTRAL, MEDLINE, Embase, LILACS, Science Citation index - expanded, the World Health Organization (WHO) International Clinical Trials Registry Platform, ClinicalTrials.gov, and the National Institutes of Health (NIH) RePORTER database to 14 January 2021. We checked the reference lists of included studies for other potentially relevant studies, and contacted researchers working in the field for unpublished and ongoing trials.
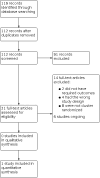
Selection criteria: We included cluster-randomized controlled trials (cRCTs) that compared ivermectin, as single or multiple doses, with a control treatment or placebo given to populations living in malaria-endemic areas, in the context of mass drug administration. Primary outcomes were prevalence of malaria parasite infection and incidence of clinical malaria in the community.
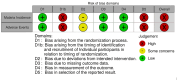
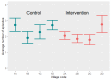
Data collection and analysis: Two review authors independently extracted data on the number of events and the number of participants in each trial arm at the time of assessment. For rate data, we noted the total time at risk in each trial arm. To assess risk of bias, we used Cochrane's RoB 2 tool for cRCTs. We documented the method of data analysis, any adjustments for clustering or other covariates, and recorded the estimate of the intra-cluster correlation (ICC) coefficient. We re-analysed the trial data provided by the trial authors to adjust for cluster effects. We used a Poisson mixed-effect model with small sample size correction, and a cluster-level analysis using the linear weighted model to adequately adjust for clustering. MAIN RESULTS: We included one cRCT and identified six ongoing trials. The included cRCT examined the incidence of malaria in eight villages in Burkina Faso, randomized to two arms. Both trial arms received a single dose of ivermectin 150 µg/kg to 200 µg/kg, together with a dose of albendazole. The villages in the intervention arm received an additional five doses of ivermectin, once every three weeks. Children were enrolled into an active cohort, in which they were repeatedly screened for malaria infection. The primary outcome was the cumulative incidence of uncomplicated malaria in a cohort of children aged five years and younger, over the 18-week study. We judged the study to be at high risk of bias, as the analysis did not account for clustering or correlation between participants in the same village. The study did not demonstrate an effect of Ivermectin on the cumulative incidence of uncomplicated malaria in the cohort of children over the 18-week study (risk ratio 0.86, 95% confidence interval (CI) 0.62 to 1.17; P = 0.2607; very low-certainty evidence).
Authors' conclusions: We are uncertain whether community administration of ivermectin has an effect on malaria transmission, based on one trial published to date.
Copyright © 2021 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
DKD has no known conflicts of interest.
RT has no known conflicts of interest.
JB is an investigator on the MASSIV and MATAMAL studies.
CL has no known conflicts of interest.
DAB has no known conflicts of interest.
JO has no known conflicts of interest.
Figures
Update of
References
References to studies included in this review
Foy 2019 {published data only}
-
- Foy BD, Alout H, Seaman JA, Rao S, Magalhaes T, Wade M, et al. RIMDAMAL I de-identified data . Data on file (as supplied 10 March 2020) .
-
- Foy BD, Rao S, Parikh S, Slater HC, Dabiré RK. Analysis of the RIMDAMAL trial - Authors' reply. Lancet 2019;394(10203):1006-7. - PubMed
-
- NCT02509481. Repeat ivermectin mass drug administrations for control of malaria: a pilot safety and efficacy study. clinicaltrials.gov/show/nct02509481 (first posted 28 July 2015).
References to studies excluded from this review
Alout 2014 {published data only}
Bockarie 1998 {published data only}
-
- Bockarie MJ, Alexander ND, Hyun P, Dimber Z, Bockarie F, Ibam E, et al. Randomised community-based trial of annual single-dose diethylcarbamazine with or without ivermectin against Wuchereria bancrofti infection in human beings and mosquitoes. Lancet 1998;351(9097):162-8. [DOI: 10.1016/S0140-6736(97)07081-5] - DOI - PubMed
Chaccour 2009 {published data only}
-
- Chaccour C. Ivermectin effect on the survival of Anopheles gambiae. Tropical Medicine and International Health 2009;14(Suppl 2):123. [DOI: 10.1111/j.1365-3156.2009.02354-1.x] - DOI
Chaccour 2010 {published data only}
-
- Chaccour C, Lines J, Whitty CJ. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. Journal of Infectious Diseases 2010;202(1):113-6. - PubMed
Derua 2015 {published data only}
de Souza 2017 {published data only}
-
- Souza DK, Ahorlu CS, Adu-Amankwah S, Otchere J, Mensah SK, Larbi IA, et al. Community-based trial of annual versus biannual single-dose ivermectin plus albendazole against Wuchereria bancrofti infection in human and mosquito populations: study protocol for a cluster randomised controlled trial. Trials 2017;18(1):448. [DOI: 10.1186/s13063-017-2196-9] - DOI - PMC - PubMed
Kobylinski 2011 {published data only}
Kobylinski 2017 {published data only}
Kositz 2017 {published data only}
-
- Kositz C, Talina J, Diau J, Asugeni R, Whitehorn C, Mabey D, et al. Incidental mosquitocidal effect of an ivermectin mass drug administration on Anopheles farauti conducted for scabies control in the Solomon Islands. Transactions of the Royal Society of Tropical Medicine and Hygiene 2017;111(3):97-101. [DOI: 10.1093/trstmh/trx025] - DOI - PMC - PubMed
Mekuriaw 2019 {published data only}
NCT02511353 {published data only}
-
- NCT02511353. Efficacy and safety of high-dose ivermectin for reducing malaria transmission: a dose finding study. clinicaltrials.gov/show/nct02511353 (first posted 30 July 2015).
Ouédraogo 2015 {published data only}
-
- Ouédraogo AL, Bastiaens GJ, Tiono AB, Guelbéogo WM, Kobylinski KC, Ouédraogo A, et al. Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double-blind, randomized, clinical trial. Clinical Infectious Diseases 2015;60(3):357-65. - PubMed
Pinilla 2018 {published data only}
-
- Pinilla YT, Lopes SCP, Sampaio VS, Andrade FS, Melo GC, Orfanó AS, et al. Promising approach to reducing Malaria transmission by ivermectin: sporontocidal effect against Plasmodium vivax in the South American vectors Anopheles aquasalis and Anopheles darlingi. PLoS Neglected Tropical Diseases 2018;12(2):e0006221. [DOI: 10.1371/journal.pntd.0006221] - DOI - PMC - PubMed
Smit 2018 {published data only}
-
- Smit MR, Ochomo EO, Aljayyoussi G, Kwambai TK, Abong'o BO, Chen T, et al. Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial. Lancet Infected Diseases 2016;18(6):615-26. - PubMed
References to ongoing studies
NCT03074435 (REACT) {published data only}
-
- NCT03074435. Insecticide resistance management in Burkina Faso and Côte d'Ivoire (REACT). https://clinicaltrials.gov/ct2/show/NCT03074435 (first posted 8 March 2017).
NCT03576313 (MASSIV) {published data only}
-
- NCT03576313. Mass Drug Administration of Ivermectin and Dihydroartemisinin-piperaquine as an Additional Intervention for Malaria Elimination (MASSIV). https://clinicaltrials.gov/ct2/show/NCT03576313 (first posted 3 July 2018).
NCT03967054 (RIMDAMAL II) {published data only}
-
- NCT03967054. Repeat Ivermectin Mass Drug Administrations for MALaria Control II (RIMDAMAL II). https://clinicaltrials.gov/ct2/show/NCT03967054 (first posted 30 May 2019).
NCT04844905 (MATAMAL) {published data only}
-
- NCT04844905. Adjunctive Ivermectin Mass Drug Administration for Malaria Control (MATAMAL). https://clinicaltrials.gov/ct2/show/NCT04844905 (first posted 14 April 2021).
PR150881 {published data only}
-
- Prachumsri J. A novel vector control measure to combat the spread of artemisinin resistance in the Greater Mekong Subregion. https://federalreporter.nih.gov/Projects/Details/?projectId=893768 (accessed 12 May 2021).
Rabinovich ongoing {published data only}
-
- Rabinovich R, Chaccour C. BOHEMIA - Broad One Health Endectocide-based Malaria Intervention in Africa. www.isglobal.org/en/-/bohemia (accessed 12 May 2021).
Additional references
Ashley 2014
Bhatt 2015
Blanford 2013
Bousema 2014
-
- Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nature Reviews Microbiology 2014;12(12):833-40. - PubMed
Boussinesq 2001
-
- Boussinesq M, Gardon M, Kamgno J, Pion SDS, Gardon-Wendel N, Chippaux J-P. Relationships between the prevalence and intensity of Loa loa infection in the central province of Cameroon. Annals of Tropical Medicine and Parasitology 2001;95(5):495-507. - PubMed
Bradley 2019
-
- Bradley J, Moulton LH, Hayes R. Analysis of the RIMDAMAL trial. Lancet 2019;394(10203):1005-6. - PubMed
Brown 2000
-
- Brown KR, Ricci FM, Ottesen EA. Ivermectin: effectiveness in lymphatic filariasis. Parasitology 2000;121(Suppl):S133-46. - PubMed
Burg 1979
Chaccour 2015
Chaccour 2017
Churcher 2016
Clements 1992
-
- Clements AN. The Biology of Mosquitoes. Development, Nutrition, and Reproduction. London: Chapman & Hall, 1992.
Cupp 2011
-
- Cupp EW, Sauerbrey M, Richards F. Elimination of human onchocerciasis: history of progress and current feasibility using ivermectin (Mectizan®) monotherapy. Acta Tropica 2011;120(Suppl 1):S100-8. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook .
Edwards 1988
-
- Edwards G, Dingsdale A, Helsby N, Orme ML, Breckenridge AM. The relative systemic availability of ivermectin after administration as capsule, tablet, and oral solution. European Journal of Clinical Pharmacology 1988;35(6):681-4. - PubMed
Eldridge 2021
-
- Eldridge S, Campbell M, Campbell M, Drahota A, Giraudeau B, Higgins J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. Available from https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool (accessed 12 May 2021).
Elkassaby 1991
-
- Elkassaby MH. Ivermectin uptake and distribution in the plasma and tissue of Sudanese and Mexican patients infected with Onchocerca volvulus. Tropical Medicine and Parasitology 1991;42(2):79-81. - PubMed
Farrar 2013
-
- Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White NJ. Manson's Tropical Diseases. 23rd revised edition. UK: Elsevier Health Sciences, 2013.
Feachem 2019
-
- Feachem RG, Chen I, Akbari O, Bertozzi-Villa A, Bhatt S, Binka F, et al. Malaria eradication within a generation: ambitious, achievable, and necessary. Lancet September 21, 2019;394(10203):1056-112. - PubMed
Foley 2000
-
- Foley DH, Bryan JH, Lawrence GW. The potential of ivermectin to control the malaria vector Anopheles farauti. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(6):625-8. - PubMed
Gardon 1997
-
- Gardon J, Gardon-Wendel N, Ngangue D, Kamgno J, Chippaux J-P, Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa. Lancet 1997;350(9070):18-20. - PubMed
González Canga 2008
GRADEpro GDT [Computer program]
-
- GRADEpro Guideline Development Tool. Version accessed 12 May 2021. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2020. Available at gradepro.org.
Graves 2018
Hay 2004
Huang 2016
-
- Huang S, Fiero MH, Bell ML. Generalized estimating equations in cluster randomized trials with a small number of clusters: review of practice and simulation study. Clinical Trials (London, England) 2016;13(4):445-9. - PubMed
Ikeda 2003
-
- Ikeda T. Pharmacological effects of ivermectin, an antiparasitic agent for intestinal strongyloidiasis: its mode of action and clinical efficacy. Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica 2003;122(6):527-38. [PMID: ] - PubMed
Ivermectin Roadmappers 2020
Kahan 2016
Kenward 1997
Killeen 2014
Leyrat 2017
-
- Leyrat C, Morgan KE, Leurent B, Kahan BC. Cluster randomized trials with a small number of clusters: which analyses should be used? International Journal of Epidemiology 2017;47(1):321–31. - PubMed
Lindblade 2013
-
- Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Review of Anti-infective Therapy 2013;11(6):623-39. - PubMed
Makunde 2003
-
- Makunde WH, Kamugisha LM, Massaga JJ, Makunde RW, Savael ZX, Akida J, et al. Treatment of co-infection with bancroftian filariasis and onchocerciasis: a safety and efficacy study of albendazole with ivermectin compared to treatment of single infection with bancroftian filariasis. Filaria Journal 2003;2(1):15. - PMC - PubMed
McGuinness 2020
Merck 2009
-
- Merck & Co. Stromectol. FDA-approved package insert. www.accessdata.fda.gov/drugsatfda_docs/label/2009/050742s026lbl.pdf (accessed 30 May 2018).
Meyers 2015
Molyneux 2003
-
- Molyneux DH, Bradley M, Hoerauf A, Kyelem D, Taylor MJ. Mass drug treatment for lymphatic filariasis and onchocerciasis. Trends in Parasitology 2003;19(11):516-22. - PubMed
Nájera 2011
Newby 2015
Nicholas 2020
Ouédraogo 2013
Paaijmans 2011
Poirot 2013
Quiñones 1997
-
- Quiñones ML, Lines JD, Thomson MC, Jawara M, Morris J, Greenwood BM. Anopheles gambiae gonotrophic cycle duration, biting and exiting behaviour unaffected by permethrin-impregnated bednets in the Gambia. Medical and Veterinary Entomology 1997;11(1):71-8. - PubMed
Riveron 2016
Russell 2013
Sinclair 2009
Slater 2020
Sougoufara 2014
Sterne 2019
-
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. - PubMed
Sy 2014
Sylla 2010
Tesh 1990
-
- Tesh RB, Guzman H. Mortality and infertility in adult mosquitoes after the ingestion of blood containing ivermectin. American Journal of Tropical Medicine and Hygiene 1990;43(3):229-33. - PubMed
Thomsen 2017
WHO 2017a
-
- World Health Organization. WHO preferred product characteristics: endectocide for malaria transmission control. apps.who.int/iris/bitstream/10665/255694/1/WHO-HTM-GMP-2017.10-eng.pdf?ua=1 (accessed 30 May 2018).
WHO 2017b
-
- World Health Organziation. Ivermectin for malaria transmission control: Technical consultation meeting report. www.who.int/malaria/mpac/mpac-sept2016-invermectin-session9.pdf?ua=1 (accessed 19 February 2021).
WHO 2019a
-
- World Health Organization. World Malaria Report 2019. www.who.int/malaria/publications/world-malaria-report-2019/en/ (accessed 06 February 2020).
WHO 2019b
-
- WHO Strategic Advisory Group on Malaria Eradication. Malaria eradication: benefits, future scenarios and feasibility. Executive summary. www.who.int/publications-detail/strategic-advisory-group-malaria-eradica... (accessed 23 August 2019).
WHO 2020
-
- World Health Organization. World Malaria Report 2020. www.who.int/malaria/publications/i/item/9789240015791 (accessed 12 May 2021).
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical