A National, Semantic-Driven, Three-Pillar Strategy to Enable Health Data Secondary Usage Interoperability for Research Within the Swiss Personalized Health Network: Methodological Study
- PMID: 34185008
- PMCID: PMC8277320
- DOI: 10.2196/27591
A National, Semantic-Driven, Three-Pillar Strategy to Enable Health Data Secondary Usage Interoperability for Research Within the Swiss Personalized Health Network: Methodological Study
Abstract
Background: Interoperability is a well-known challenge in medical informatics. Current trends in interoperability have moved from a data model technocentric approach to sustainable semantics, formal descriptive languages, and processes. Despite many initiatives and investments for decades, the interoperability challenge remains crucial. The need for data sharing for most purposes ranging from patient care to secondary uses, such as public health, research, and quality assessment, faces unmet problems.
Objective: This work was performed in the context of a large Swiss Federal initiative aiming at building a national infrastructure for reusing consented data acquired in the health care and research system to enable research in the field of personalized medicine in Switzerland. The initiative is the Swiss Personalized Health Network (SPHN). This initiative is providing funding to foster use and exchange of health-related data for research. As part of the initiative, a national strategy to enable a semantically interoperable clinical data landscape was developed and implemented.
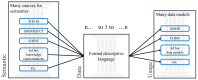
Methods: A deep analysis of various approaches to address interoperability was performed at the start, including large frameworks in health care, such as Health Level Seven (HL7) and Integrating Healthcare Enterprise (IHE), and in several domains, such as regulatory agencies (eg, Clinical Data Interchange Standards Consortium [CDISC]) and research communities (eg, Observational Medical Outcome Partnership [OMOP]), to identify bottlenecks and assess sustainability. Based on this research, a strategy composed of three pillars was designed. It has strong multidimensional semantics, descriptive formal language for exchanges, and as many data models as needed to comply with the needs of various communities.
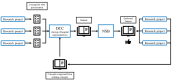
Results: This strategy has been implemented stepwise in Switzerland since the middle of 2019 and has been adopted by all university hospitals and high research organizations. The initiative is coordinated by a central organization, the SPHN Data Coordination Center of the SIB Swiss Institute of Bioinformatics. The semantics is mapped by domain experts on various existing standards, such as Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT), Logical Observation Identifiers Names and Codes (LOINC), and International Classification of Diseases (ICD). The resource description framework (RDF) is used for storing and transporting data, and to integrate information from different sources and standards. Data transformers based on SPARQL query language are implemented to convert RDF representations to the numerous data models required by the research community or bridge with other systems, such as electronic case report forms.
Conclusions: The SPHN strategy successfully implemented existing standards in a pragmatic and applicable way. It did not try to build any new standards but used existing ones in a nondogmatic way. It has now been funded for another 4 years, bringing the Swiss landscape into a new dimension to support research in the field of personalized medicine and large interoperable clinical data.
Keywords: clinical data reuse; interoperability; personalized medicine.
©Christophe Gaudet-Blavignac, Jean Louis Raisaro, Vasundra Touré, Sabine Österle, Katrin Crameri, Christian Lovis. Originally published in JMIR Medical Informatics (https://medinform.jmir.org), 24.06.2021.
Conflict of interest statement
Conflicts of Interest: CL is the Editor-in-Chief of this journal (JMIR Medical Informatics).
Figures
References
-
- International Classification of Diseases, 11th Revision (ICD-11) World Health Organization. [2021-06-09]. http://www.who.int/classifications/icd/en/
-
- Health Level Seven International-Homepage. HL7 International. [2021-06-09]. http://www.hl7.org/
-
- Bhattacharyya SB. Introduction to SNOMED CT. Singapore: Springer; 2016. SNOMED CT History and IHTSDO.
-
- Integrating the Healthcare Enterprise (IHE) IHE International. [2021-06-09]. https://www.ihe.net/
LinkOut - more resources
Full Text Sources
Research Materials

