The role of metabolic reprogramming in tubular epithelial cells during the progression of acute kidney injury
- PMID: 34185125
- PMCID: PMC11073237
- DOI: 10.1007/s00018-021-03892-w
The role of metabolic reprogramming in tubular epithelial cells during the progression of acute kidney injury
Abstract
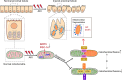
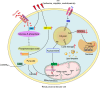
Acute kidney injury (AKI) is one of the most common clinical syndromes. AKI is associated with significant morbidity and subsequent chronic kidney disease (CKD) development. Thus, it is urgent to develop a strategy to hinder AKI progression. Renal tubules are responsible for the reabsorption and secretion of various solutes and the damage to this part of the nephron is a key mediator of AKI. As we know, many common renal insults primarily target the highly metabolically active proximal tubular cells (PTCs). PTCs are the most energy-demanding cells in the kidney. The ATP that they use is mostly produced in their mitochondria by fatty acid β-oxidation (FAO). But, when PTCs face various biological stresses, FAO will shut down for a time that outlives injury. Recent studies have suggested that surviving PTCs can adapt to FAO disruption by increasing glycolysis when facing metabolic constraints, although PTCs do not perform glycolysis in a normal physiological state. Enhanced glycolysis in a short period compensates for impaired energy production and exerts partial renal-protective effects, but its long-term effect on renal function and AKI progression is not promising. Deranged FAO and enhanced glycolysis may contribute to the AKI to CKD transition through different molecular biological mechanisms. In this review, we concentrate on the recent pathological findings of AKI with regards to the metabolic reprogramming in PTCs, confirming that targeting metabolic reprogramming represents a potentially effective therapeutic strategy for the progression of AKI.
Keywords: Acute kidney injury; Fatty acid oxidation; Glycolysis; Metabolic reprogramming; Proximal tubular epithelial cells.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, Toussaint ND, Bellomo R. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160–172. doi: 10.1016/j.kint.2018.08.036. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

