Cyclic tensile strain affects the response of human periodontal ligament stromal cells to tumor necrosis factor-α
- PMID: 34185172
- PMCID: PMC8791913
- DOI: 10.1007/s00784-021-04039-8
Cyclic tensile strain affects the response of human periodontal ligament stromal cells to tumor necrosis factor-α
Abstract
Objectives: Orthodontic treatment in adult patients predisposed to mild or severe periodontal disease is challenging for orthodontists. Orthodontic malpractice or hyper-occlusal forces may aggravate periodontitis-induced destruction of periodontal tissues, but the specific mechanism remains unknown. In the present study, the combined effect of mechanical stress and tumor necrosis factor (TNF)-α on the inflammatory response in human periodontal ligament stromal cells (hPDLSCs) was investigated.
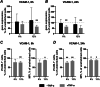
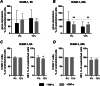
Materials and methods: hPDLSCs from 5 healthy donors were treated with TNF-α and/or subjected to cyclic tensile strain (CTS) of 6% or 12% elongation with 0.1 Hz for 6- and 24 h. The gene expression of interleukin (IL)-6, IL-8 and cell adhesion molecules VCAM and ICAM was analyzed by qPCR. The protein levels of IL-6 and IL-8 in conditioned media was measured by ELISA. The surface expression of VCAM-1 and ICAM-1 was quantified by immunostaining followed by flow cytometry analysis.
Results: TNF-α-induced IL-6 gene and protein expression was inhibited by CTS, whereas TNF-α-induced IL-8 expression was decreased at mRNA expression level but enhanced at the protein level in a magnitude-dependent manner. CTS downregulated the gene expression of VCAM-1 and ICAM-1 under TNF-α stimulation, but the downregulation of the surface expression analyzed by flow cytometry was observed chiefly for VCAM-1.
Conclusions: Our findings show that mechanical force differentially regulates TNF-α-induced expression of inflammatory mediators and adhesion molecules at the early stage of force application. The effect of cyclic tensile strain is complex and could be either anti-inflammatory or pro-inflammatory depending on the type of pro-inflammatory mediators and force magnitude.
Clinical relevance: Orthodontic forces regulate the inflammatory mediators of periodontitis. The underlying mechanism may have significant implications for future strategies of combined periodontal and orthodontic treatment.
Keywords: Human periodontal ligament stromal cells; Inflammatory cytokine; Mechanical loading; Orthodontic force; Periodontitis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Proffit WR (2018) Contemporary orthodontics. 6th edition. edn. Elsevier, Philadelphia, IL
-
- Behm C, Nemec M, Blufstein A, Schubert M, Rausch-Fan X, Andrukhov O, Jonke E. Interleukin-1beta induced matrix metalloproteinase expression in human periodontal ligament-derived mesenchymal stromal cells under in vitro simulated static orthodontic forces. Int J Mol Sci. 2021;22:3. doi: 10.3390/ijms22031027. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

