Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2020 for the Clinical Practice of Hereditary Colorectal Cancer
- PMID: 34185173
- PMCID: PMC8286959
- DOI: 10.1007/s10147-021-01881-4
Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2020 for the Clinical Practice of Hereditary Colorectal Cancer
Abstract
Hereditary colorectal cancer (HCRC) accounts for < 5% of all colorectal cancer cases. Some of the unique characteristics commonly encountered in HCRC cases include early age of onset, synchronous/metachronous cancer occurrence, and multiple cancers in other organs. These characteristics necessitate different management approaches, including diagnosis, treatment or surveillance, from sporadic colorectal cancer management. There are two representative HCRC, named familial adenomatous polyposis and Lynch syndrome. Other than these two HCRC syndromes, related disorders have also been reported. Several guidelines for hereditary disorders have already been published worldwide. In Japan, the first guideline for HCRC was prepared by the Japanese Society for Cancer of the Colon and Rectum (JSCCR), published in 2012 and revised in 2016. This revised version of the guideline was immediately translated into English and published in 2017. Since then, several new findings and novel disease concepts related to HCRC have been discovered. The currently diagnosed HCRC rate in daily clinical practice is relatively low; however, this is predicted to increase in the era of cancer genomic medicine, with the advancement of cancer multi-gene panel testing or whole genome testing, among others. Under these circumstances, the JSCCR guidelines 2020 for HCRC were prepared by consensus among members of the JSCCR HCRC Guideline Committee, based on a careful review of the evidence retrieved from literature searches, and considering the medical health insurance system and actual clinical practice settings in Japan. Herein, we present the English version of the JSCCR guidelines 2020 for HCRC.
Keywords: Familial adenomatous polyposis; Guidelines; Hereditary colorectal cancer; Lynch syndrome.
© 2021. The Author(s).
Conflict of interest statement
All members with the exception of the discussion chair (committee chair) voted in all CQs. None of the committee members reported on economic COIs, determined according to the rules of the JSCCR. Also, none of the commissioners had academic COIs for CQs. Therefore, all of the committee members except the discussion chair voted in all CQs. Conflict of interest statements according to rules of the International Journal of Clinical Oncology was as follows. Naohiro Tomita received a research grant from Taiho pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., and Sysmex Co.; Hideyuki Ishida received a research grant from Chugai Pharmaceutical Co., Ltd.,Eli Lilly Japan K.K. and Taiho pharmaceutical Co., Ltd.; Kiwamu Akagi received honoraria from MSD Co., Ltd., Taiho pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd., received a research grant from Ono Pharmaceutical Co. Ltd. and FALCO Biosystems Co., Ltd.; Michio Itabashi received a research grant from Taiho pharmaceutical Co., Ltd., Pfizer Japan, Inc., Astellas Pharma, Inc., Chugai Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd. Other authors declare that they have no conflict of interest.
Figures



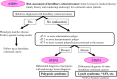
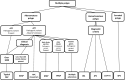





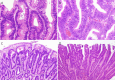



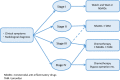
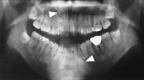



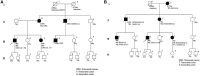
 : asymptomatic/presymptomatic individual with pathogenic variant.
: asymptomatic/presymptomatic individual with pathogenic variant.  : personal numbers can be assigned to the upper right of individuals
: personal numbers can be assigned to the upper right of individuals
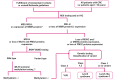

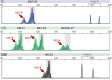


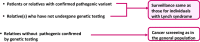



References
-
- Japanese Society for the Cancer of the Colon and Rectum . JSCCR guidelines 2019 for the treatment of colorectal cancer. Tokyo: Kanehara & Co. Ltd; 2019.
LinkOut - more resources
Full Text Sources

