BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies
- PMID: 34185336
- PMCID: PMC8420332
- DOI: 10.1002/ajh.26284
BNT162b2 COVID-19 vaccine is significantly less effective in patients with hematologic malignancies
Abstract
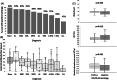
Patients with hematologic malignancies have an increased risk of severe COVID-19 infection. Vaccination against COVID-19 is especially important in these patients, but whether they develop an immune response following vaccination is unknown. We studied serologic responses to the BNT162b2 vaccine in this population. A lower proportion of patients were seropositive following vaccination (75%) than in a comparison group (99%; p < 0.001), and median (interquartile range [IQR]) antibody titers in patients were lower (90 [12.4-185.5] and 173 [133-232] AU/ml, respectively; p < 0.001). Older age, higher lactate dehydrogenase, and number of treatment lines correlated with lower seropositivity likelihood and antibody titers, while absolute lymphocyte count, globulin level, and time from last treatment to vaccination correlated with higher seropositivity likelihood and antibody titers. Chronic lymphocytic leukemia patients had the lowest seropositivity rate followed by indolent lymphoma. Patients recently treated with chemo-immunotherapy, anti-CD20 antibodies, BCL2, BTK or JAK2 inhibitors had significantly less seropositive responses and lower median (IQR) antibody titers (29%, 1.9 [1.9-12] AU/ml; 0%, 1.9 [1.9-1.9] AU/ml; 25%, 1.9 [1.9-25] AU/ml; 40%, 1.9 [1.9-92.8] AU/ml; and 42%, 10.9 [5.7-66.4] AU/ml, respectively; p < 0.001). Serological response to BNT162b2 vaccine in patients with hematologic malignancies is considerably impaired, and they could remain at risk for severe COVID-19 infection and death.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
All authors declare there are no relevant disclosures or conflicting financial interests.
Figures

References
-
- COVID‐19 dashboard by the Center for Systems Science and Engineering at John Hopkins University of Medicine. https://coronavirus.jhu.edu/map.html. Accessed April 20, 2021.
-
- Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID‐19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737‐e745. 10.1016/S2352-3026(20)30251-9 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
