Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial
- PMID: 34185551
- PMCID: PMC8445562
- DOI: 10.1200/JCO.21.00163
Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial
Abstract
Purpose: Donafenib, a novel multikinase inhibitor and a deuterated sorafenib derivative, has shown efficacy in phase Ia and Ib hepatocellular carcinoma (HCC) studies. This study compared the efficacy and safety of donafenib versus sorafenib as first-line therapy for advanced HCC.
Patients and methods: This open-label, randomized, parallel-controlled, multicenter phase II-III trial enrolled patients with unresectable or metastatic HCC, a Child-Pugh score ≤ 7, and no prior systemic therapy from 37 sites across China. Patients were randomly assigned (1:1) to receive oral donafenib (0.2 g) or sorafenib (0.4 g) twice daily until intolerable toxicity or disease progression. The primary end point was overall survival (OS), tested for noninferiority and superiority. Efficacy was primarily assessed in the full analysis set (FAS), and safety was assessed in all treated patients.
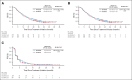
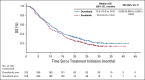
Results: Between March 21, 2016, and April 16, 2018, 668 patients (intention-to-treat) were randomly assigned to donafenib and sorafenib treatment arms; the FAS included 328 and 331 patients, respectively. Median OS was significantly longer with donafenib than sorafenib treatment (FAS; 12.1 v 10.3 months; hazard ratio, 0.831; 95% CI, 0.699 to 0.988; P = .0245); donafenib also exhibited superior OS outcomes versus sorafenib in the intention-to-treat population. The median progression-free survival was 3.7 v 3.6 months (P = .0570). The objective response rate was 4.6% v 2.7% (P = .2448), and the disease control rate was 30.8% v 28.7% (FAS; P = .5532). Drug-related grade ≥ 3 adverse events occurred in significantly fewer patients receiving donafenib than sorafenib (125 [38%] v 165 [50%]; P = .0018).
Conclusion: Donafenib showed superiority over sorafenib in improving OS and has favorable safety and tolerability in Chinese patients with advanced HCC, showing promise as a potential first-line monotherapy for these patients.
Conflict of interest statement
Figures



References
-
- Bray F Ferlay J Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 - PubMed
-
- Chen W Zheng R Baade PD, et al. : Cancer statistics in China, 2015. CA Cancer J Clin 66:115-132, 2016 - PubMed
-
- European Association for the Study of the Liver: EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182-236, 2018 - PubMed
-
- Fan JH Wang JB Jiang Y, et al. : Attributable causes of liver cancer mortality and incidence in China. Asian Pac J Cancer Prev 14:7251-7256, 2013 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

