IL-13 is a driver of COVID-19 severity
- PMID: 34185704
- PMCID: PMC8410056
- DOI: 10.1172/jci.insight.150107
IL-13 is a driver of COVID-19 severity
Abstract
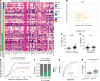
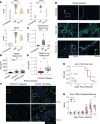
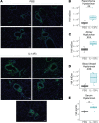
Immune dysregulation is characteristic of the more severe stages of SARS-CoV-2 infection. Understanding the mechanisms by which the immune system contributes to COVID-19 severity may open new avenues to treatment. Here, we report that elevated IL-13 was associated with the need for mechanical ventilation in 2 independent patient cohorts. In addition, patients who acquired COVID-19 while prescribed Dupilumab, a mAb that blocks IL-13 and IL-4 signaling, had less severe disease. In SARS-CoV-2-infected mice, IL-13 neutralization reduced death and disease severity without affecting viral load, demonstrating an immunopathogenic role for this cytokine. Following anti-IL-13 treatment in infected mice, hyaluronan synthase 1 (Has1) was the most downregulated gene, and accumulation of the hyaluronan (HA) polysaccharide was decreased in the lung. In patients with COVID-19, HA was increased in the lungs and plasma. Blockade of the HA receptor, CD44, reduced mortality in infected mice, supporting the importance of HA as a pathogenic mediator. Finally, HA was directly induced in the lungs of mice by administration of IL-13, indicating a new role for IL-13 in lung disease. Understanding the role of IL-13 and HA has important implications for therapy of COVID-19 and, potentially, other pulmonary diseases. IL-13 levels were elevated in patients with severe COVID-19. In a mouse model of the disease, IL-13 neutralization reduced the disease and decreased lung HA deposition. Administration of IL-13-induced HA in the lung. Blockade of the HA receptor CD44 prevented mortality, highlighting a potentially novel mechanism for IL-13-mediated HA synthesis in pulmonary pathology.
Keywords: COVID-19; Cytokines; Immunology; Innate immunity; Th2 response.
Figures


Update of
-
IL-13 is a driver of COVID-19 severity.medRxiv [Preprint]. 2021 Mar 1:2020.06.18.20134353. doi: 10.1101/2020.06.18.20134353. medRxiv. 2021. Update in: JCI Insight. 2021 Aug 9;6(15):150107. doi: 10.1172/jci.insight.150107. PMID: 33688686 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

