Neurotoxicity of methamphetamine: Main effects and mechanisms
- PMID: 34186102
- PMCID: PMC8338805
- DOI: 10.1016/j.expneurol.2021.113795
Neurotoxicity of methamphetamine: Main effects and mechanisms
Abstract
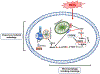
Methamphetamine (METH) is an illicit psychostimulant that is abused throughout the world. METH addiction is also a major public health concern and the abuse of large doses of the drug is often associated with serious neuropsychiatric consequences that may include agitation, anxiety, hallucinations, paranoia, and psychosis. Some human methamphetamine users can also suffer from attention, memory, and executive deficits. METH-associated neurological and psychiatric complications might be related, in part, to METH-induced neurotoxic effects. Those include altered dopaminergic and serotonergic functions, neuronal apoptosis, astrocytosis, and microgliosis. Here we have endeavored to discuss some of the main effects of the drug and have presented the evidence supporting certain of the molecular and cellular bases of METH neurotoxicity. The accumulated evidence suggests the involvement of transcription factors, activation of dealth pathways that emanate from mitochondria and endoplasmic reticulum (ER), and a role for neuroinflammatory mechanisms. Understanding the molecular processes involved in METH induced neurotoxicity should help in developing better therapeutic approaches that might also serve to attenuate or block the biological consequences of use of large doses of the drug by some humans who meet criteria for METH use disorder.
Keywords: Autophagy; Bcl2; Cell death; ER stress; Mitochondrial cascade; Neuroinflammation; Transcription factors.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Figures



References
-
- Achat-Mendes C, Ali SF, Itzhak Y (2005) Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive pavlovian conditioning in mice. Neuropsychopharmacology 30:1128–1137. - PubMed
-
- Ando K, Johanson CE, Seiden LS, Schuster CR (1985) Sensitivity changes to dopaminergic agents in fine moto control of rhesus monkeys after repeated methamphetamine administration. Pharmacology Biochemistry and Behavior 22:737–743. - PubMed
-
- Ares-Santos S, Granado N, Moratalla R (2013) The role of dopamine receptors in the neurotoxicity of methamphetamine. J Intern Med 273:437–453. - PubMed
-
- Ares-Santos S, Granado N, Oliva I, Martin ED, Colado MI, Moratalla R (2012) Dopamine D (1) receptor deletion strongly reduces neurotoxic effects of methamphetamine. Neurobiol Dis 45:810–820. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

