Prospective multicenter evaluation of real time PCR Kit prototype for early diagnosis of congenital Chagas disease
- PMID: 34186488
- PMCID: PMC8243352
- DOI: 10.1016/j.ebiom.2021.103450
Prospective multicenter evaluation of real time PCR Kit prototype for early diagnosis of congenital Chagas disease
Abstract
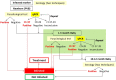
Background: Current algorithm for Congenital Chagas Disease (cCD) diagnosis is unsatisfactory due to low sensitivity of the parasitological methods. Moreover, loss to follow-up precludes final serodiagnosis after nine months of life in many cases. A duplex TaqMan qPCR kit for Trypanosoma cruzi DNA amplification was prospectively evaluated in umbilical cord (UCB) and peripheral venous blood (PVB) of infants born to CD mothers at endemic and non-endemic sites of Argentina.
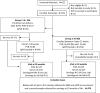
Methods: We enrolled and followed-up 370 infants; qPCR was compared to gold-standard cCD diagnosis following studies of diagnostic accuracy guidelines.
Findings: Fourteen infants (3·78%) had cCD. The qPCR sensitivity and specificity were higher in PVB (72·73%, 99·15% respectively) than in UCB (66·67%, 96·3%). Positive and negative predictive values were 80 and 98·73% and 50 and 98·11% for PVB and UCB, respectively. The Areas under the Curve (AUC) of ROC analysis for qPCR and micromethod (MM) were 0·81 and 0·67 in UCB and 0·86 and 0·68 in PVB, respectively. Parasitic loads ranged from 37·5 to 23,709 parasite equivalents/mL. Discrete typing Unit Tc V was identified in five cCD patients and in six other cCD cases no distinction among Tc II, Tc V or Tc VI was achieved.
Interpretation: This first prospective field study demonstrated that qPCR was more sensitive than MM for early cCD detection and more accurate in PVB than in UCB. Its use, as an auxiliary diagnostic tool to MM will provide more accurate records on cCD incidence.
Funding: FITS SALUD 001-CHAGAS (FONARSEC, MINCyT, Argentina) to the Public-Private Consortium (INGEBI-CONICET, INP-ANLIS MALBRAN and Wiener Laboratories); ERANET-LAC-HD 328 to AGS and PICT 2015-0074 (FONCYT, MinCyT) to AGS and FA.
Keywords: Congenital Chagas disease; Discrete typing unit; Early diagnosis; Parasitic load; Real Time PCR; Trypanosoma cruzi.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None of the authors received payment or service from a third part at any time. None of the authors have any patents relevant to the work.
Figures


References
-
- Bisio M, Seidenstein ME, Burgos JM. Urbanization of congenital transmission of Trypanosoma cruzi: prospective polymerase chain reaction study in pregnancy. Trans R Soc Trop Med Hyg. 2011;105(10):543–549. - PubMed
-
- Bittencourt AL. Possible risk factors for vertical transmission of Chagas' disease. Rev Inst Med Trop Sao Paulo. 1992;34(5):403–408. Review. - PubMed
-
- Sanchez Negrete O, Mora MC, Basombrio MA. High prevalence of congenital Trypanosoma cruzi infection and family clustering in Salta, Argentina. Pediatrics. 2005;115:e668–e672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

