Clinical performance evaluation of SARS-CoV-2 rapid antigen testing in point of care usage in comparison to RT-qPCR
- PMID: 34186490
- PMCID: PMC8234263
- DOI: 10.1016/j.ebiom.2021.103455
Clinical performance evaluation of SARS-CoV-2 rapid antigen testing in point of care usage in comparison to RT-qPCR
Abstract
Background: Antigen rapid diagnostic tests (RDT) for SARS-CoV-2 are fast, broadly available, and inexpensive. Despite this, reliable clinical performance data from large field studies is sparse.
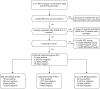
Methods: In a prospective performance evaluation study, RDT from three manufacturers (NADAL®, Panbio™, MEDsan®, conducted on different samples) were compared to quantitative reverse transcription polymerase chain reaction (RT-qPCR) in 5 068 oropharyngeal swabs for detection of SARS-CoV-2 in a hospital setting. Viral load was derived from standardised RT-qPCR Cycle threshold (Ct) values. The data collection period ranged from November 12, 2020 to February 28, 2021.
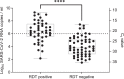
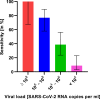
Findings: The sensitivity of RDT compared to RT-qPCR was 42·57% (95% CI 33·38%-52·31%). The specificity was 99·68% (95% CI 99·48%-99·80%). Sensitivity declined with decreasing viral load from 100% in samples with a deduced viral load of ≥108 SARS-CoV-2 RNA copies per ml to 8·82% in samples with a viral load lower than 104 SARS-CoV-2 RNA copies per ml. No significant differences in sensitivity or specificity could be observed between samples with and without spike protein variant B.1.1.7. The NPV in the study cohort was 98·84%; the PPV in persons with typical COVID-19 symptoms was 97·37%, and 28·57% in persons without or with atypical symptoms.
Interpretation: RDT are a reliable method to diagnose SARS-CoV-2 infection in persons with high viral load. RDT are a valuable addition to RT-qPCR testing, as they reliably detect infectious persons with high viral loads before RT-qPCR results are available.
Funding: German Federal Ministry for Education and Science (BMBF), Free State of Bavaria.
Keywords: Antigen rapid diagnostic test; COVID-19; Clinical evaluation; PCR; Performance evaluation; SARS-CoV-2.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None of the authors has any conflict of interest.
Figures

Comment in
-
COVID-19 rapid antigen testing strategies require careful evaluation.EBioMedicine. 2021 Aug;70:103491. doi: 10.1016/j.ebiom.2021.103491. Epub 2021 Jul 17. EBioMedicine. 2021. PMID: 34284175 Free PMC article. No abstract available.
References
-
- Krüger S., Leskien M., Schuller P. Performance and feasibility of universal PCR admission screening for SARS-CoV-2 in a German tertiary care hospital. J Med Virol. 2021;93(5):28890–28898. - PubMed
-
- Robert Koch-Institut . 2021. COVID-19-Dashboard.https://experience.arcgis.com/experience/478220a4c454480e823b17327b2bf1d4
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

