Effect of Liraglutide on Arterial Inflammation Assessed as [18F]FDG Uptake in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 34187185
- PMCID: PMC8300846
- DOI: 10.1161/CIRCIMAGING.120.012174
Effect of Liraglutide on Arterial Inflammation Assessed as [18F]FDG Uptake in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Background: The mechanism behind the cardiovascular protection observed with human GLP-1 RA (glucagon-like peptide-1 receptor agonists) in type 2 diabetes is unknown. We hypothesized that treatment with the GLP-1 RA liraglutide had a positive effect on vascular inflammation.
Methods: LIRAFLAME (Effect of liraglutide on vascular inflammation in type-2 diabetes: A randomized, placebocontrolled, double-blind, parallel clinical PET/CT trial) was a double-blind, randomized controlled trial performed at a single university hospital clinic in Denmark. Patients with type 2 diabetes were via computer-generated randomization list assigned (1:1) liraglutide up to 1.8 mg or placebo once daily for 26 weeks. The primary end point was change in vascular inflammation over 26 weeks assessed by [18F]-fluorodeoxyglucose positron emission tomography/computed tomography. Analyses were based on intention-to-treat. Key secondary outcomes included change in other indices of atherosclerosis.
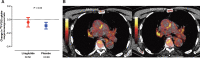
Results: Between October 26, 2017, and August 16, 2019, 147 patients were screened and 102 were randomly assigned to liraglutide (n=51) or placebo (n=51) and 99 (97%) completed the trial. Change in the [18F]-fluorodeoxyglucose positron emission tomography measure of vascular inflammation (active-segment target-to-background ratio) did not differ between treatment groups: change from baseline to 26 weeks was -0.04 (95% CI, -0.17 to 0.08) in the liraglutide group compared with -0.09 (-0.19 to 0.01) in the placebo group (mean difference, 0.05 [95% CI, -0.11 to 0.21], P=0.53). Secondary analyses restricted to [18F]-fluorodeoxyglucose positron emission tomography of the carotid arteries as well as other indices of atherosclerosis confirmed the primary result. We performed an explorative analysis of interaction between treatment group and history of cardiovascular disease (P=0.052).
Conclusions: In this low to moderate risk population with type 2 diabetes, liraglutide did not change vascular inflammation assessed as [18F]-fluorodeoxyglucose uptake compared with placebo. An explorative analysis indicated a possible effect in persons with history of cardiovascular disease, in line with current guidelines where liraglutide is recommended to patients with history of cardiovascular disease. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03449654.
Keywords: Type 2 Diabetes; atherosclerosis; cardiovascular diseases; carotid arteries; glucagon-like peptide 1; inflammation.
Figures

References
-
- Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. ; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–322. doi: 10.1056/NEJMoa1603827 - PMC - PubMed
-
- Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, et al. ; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375:1834–1844. doi: 10.1056/NEJMoa1607141 - PubMed
-
- Hernandez AF, Green JB, Janmohamed S, D’Agostino RB, Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, et al. ; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018; 392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X - PubMed
-
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, et al. ; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019; 394:121–130. doi: 10.1016/S0140-6736(19)31149-3 - PubMed
-
- Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D’Alessio DA, Davies MJ. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020; 43:487–493. doi: 10.2337/dci19-0066 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

