AMPK hyperactivation promotes dendrite retraction, synaptic loss, and neuronal dysfunction in glaucoma
- PMID: 34187514
- PMCID: PMC8243567
- DOI: 10.1186/s13024-021-00466-z
AMPK hyperactivation promotes dendrite retraction, synaptic loss, and neuronal dysfunction in glaucoma
Abstract
Background: The maintenance of complex dendritic arbors and synaptic transmission are processes that require a substantial amount of energy. Bioenergetic decline is a prominent feature of chronic neurodegenerative diseases, yet the signaling mechanisms that link energy stress with neuronal dysfunction are poorly understood. Recent work has implicated energy deficits in glaucoma, and retinal ganglion cell (RGC) dendritic pathology and synapse disassembly are key features of ocular hypertension damage.
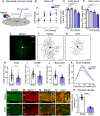
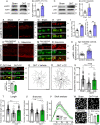
Results: We show that adenosine monophosphate-activated protein kinase (AMPK), a conserved energy biosensor, is strongly activated in RGC from mice with ocular hypertension and patients with primary open angle glaucoma. Our data demonstrate that AMPK triggers RGC dendrite retraction and synapse elimination. We show that the harmful effect of AMPK is exerted through inhibition of the mammalian target of rapamycin complex 1 (mTORC1). Attenuation of AMPK activity restores mTORC1 function and rescues dendrites and synaptic contacts. Strikingly, AMPK depletion promotes recovery of light-evoked retinal responses, improves axonal transport, and extends RGC survival.
Conclusions: This study identifies AMPK as a critical nexus between bioenergetic decline and RGC dysfunction during pressure-induced stress, and highlights the importance of targeting energy homeostasis in glaucoma and other neurodegenerative diseases.
Keywords: Adenosine monophosphate-activated protein kinase; Glaucoma; Mammalian target of rapamycin; Metabolic stress; Neurodegeneration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75(5):762-77. 10.1016/j.neuron.2012.08.019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

