Management of allergic rhinitis with leukotriene receptor antagonists versus selective H1-antihistamines: a meta-analysis of current evidence
- PMID: 34187561
- PMCID: PMC8243504
- DOI: 10.1186/s13223-021-00564-z
Management of allergic rhinitis with leukotriene receptor antagonists versus selective H1-antihistamines: a meta-analysis of current evidence
Abstract
Background: Inconsistencies remain regarding the effectiveness and safety of leukotriene receptor antagonists (LTRAs) and selective H1-antihistamines (SAHs) for allergic rhinitis (AR). A meta-analysis of randomized controlled trials (RCTs) was conducted to compare the medications.
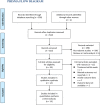
Methods: Relevant head-to-head comparative RCTs were retrieved by searching the PubMed, Embase, and Cochrane's Library databases from inception to April 20, 2020. A random-effects model was applied to pool the results. Subgroup analyses were performed for seasonal and perennial AR.
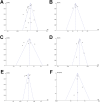
Results: Fourteen RCTs comprising 4458 patients were included. LTRAs were inferior to SAHs in terms of the daytime nasal symptoms score (mean difference [MD]: 0.05, 95% confidence interval [CI] 0.02 to 0.08, p = 0.003, I2 = 89%) and daytime eye symptoms score (MD: 0.05, 95% CI 0.01 to 0.08, p = 0.009, I2 = 89%), but were superior in terms of the nighttime symptoms score (MD: - 0.04, 95% CI - 0.06 to - 0.02, p < 0.001, I2 = 85%). The effects of the two treatments on the composite symptom score (MD: 0.02, 95% CI - 0.02 to 0.05, p = 0.30, I2 = 91%) and rhinoconjunctivitis quality-of-life questionnaire (RQLQ) (MD: 0.01, 95% CI - 0.05 to 0.07, p = 0.71, I2 = 99%) were similar. Incidences of adverse events were comparable (odds ratio [OR]: 0.97, 95% CI 0.75 to 1.25, p = 0.98, I2 = 0%). These results were mainly obtained from studies on seasonal AR. No significant publication bias was detected.
Conclusions: Although both treatments are safe and effective in improving the quality of life (QoL) in AR patients, LTRAs are more effective in improving nighttime symptoms but less effective in improving daytime nasal symptoms compared to SAHs.
Keywords: Allergic rhinitis; H1-antihistamines; Leukotriene receptor antagonists; Meta-analysis; Randomized controlled trials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

